Volume 28, Number 7—July 2022
Research
Self-Reported and Physiologic Reactions to Third BNT162b2 mRNA COVID-19 (Booster) Vaccine Dose
Figure 1
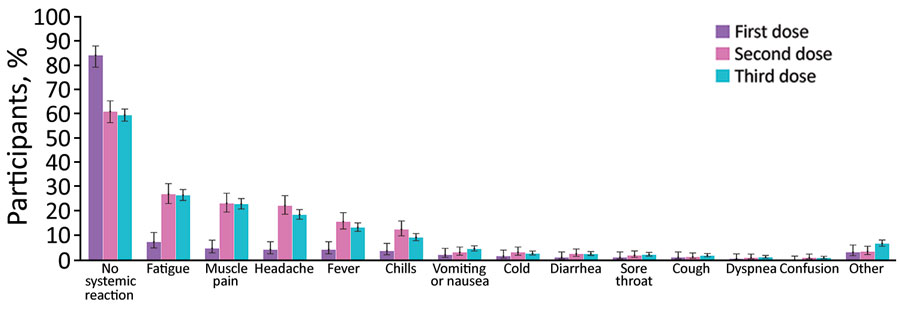
Figure 1. Reactions reported by participants through a mobile application for self-reported and physiologic reactions to BNT162b2 (Pfizer, https://www.pfizer.com) mRNA coronavirus disease vaccine doses. Error bars indicate 90% CIs.
1These authors contributed equally to this article.
Page created: April 12, 2022
Page updated: June 18, 2022
Page reviewed: June 18, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.