Lack of Evidence for Ribavirin Treatment of Lassa Fever in Systematic Review of Published and Unpublished Studies1
Hung-Yuan Cheng, Clare E. French, Alex P. Salam, Sarah Dawson, Alexandra McAleenan, Luke A. McGuinness, Jelena Savović, Peter W. Horby, and Jonathan A.C. Sterne
Author affiliations: Population Health Sciences, University of Bristol, Bristol, UK (H.-Y. Cheng, C.E. French, S. Dawson, A. McAleenan, L.A. McGuinness, J. Savović, J.A.C. Sterne); National Institute for Health and Care Research (NIHR) Health Protection Research Unit in Behavioural Science and Evaluation, University of Bristol, Bristol (C.E. French); United Kingdom Public Health Rapid Support Team, London, UK (A.P. Salam); Pandemic Sciences Centre, University of Oxford, Oxford, UK (A.P. Salam, P.W. Horby); NIHR Applied Research Collaboration West, Bristol (J. Savović); International Severe Acute Respiratory and Emerging Infections Consortium, Oxford (P. Horby); NIHR Bristol Biomedical Research Centre, Bristol (J.A.C. Sterne); Health Data Research UK South West, Bristol (J.A.C. Sterne)
Main Article
Figure 2
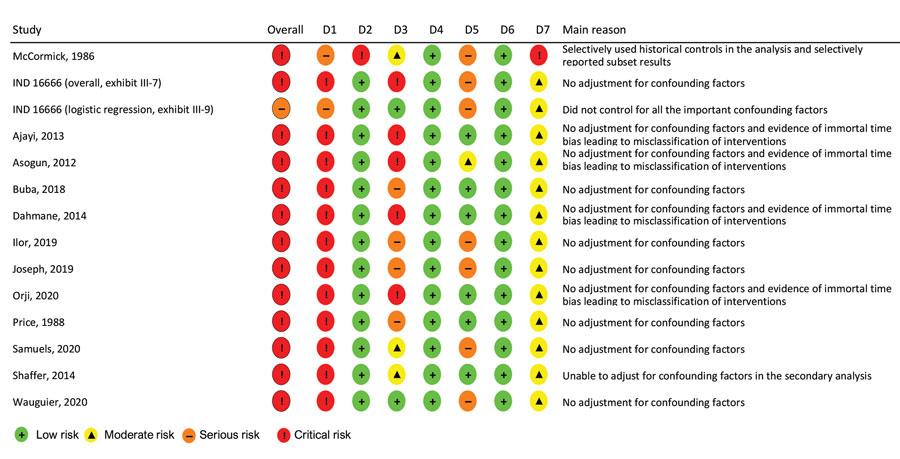
Figure 2. Summary of risk for bias assessment for a systematic review of published and unpublished studies for evidence for ribavirin treatment of Lassa fever. Bias categories: D1, bias due to confounding; D2, bias in selection of participants into the study; D3, bias in classification of interventions; D4, bias due to deviations from intended interventions; D5, bias due to missing data; D6, bias in measurement of outcomes; D7, bias in selection of the reported result. *IND 16666, unpublished study requested by P.W.H. through the US Freedom of Information Act (Birch & Davis Associates and Sherikon Inc., US Army Medical Research and Development Command, unpub. data, https://media.tghn.org/medialibrary/2019/03/Responsive_Documents_of_Peter_Horby.pdf.pdf; G.V. Ludwig, pers. comm., 2019 March 4, https://media.tghn.org/medialibrary/2019/03/Dr._Ludwig_memo.pdf). †M.-L. Orji et al., unpub. data, https://doi.org/10.20944/preprints202005.0269.v1.
Main Article
Page created: June 14, 2022
Page updated: July 24, 2022
Page reviewed: July 24, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
