Volume 28, Number 8—August 2022
Dispatch
Child Melioidosis Deaths Caused by Burkholderia pseudomallei–Contaminated Borehole Water, Vietnam, 2019
Abstract
Within 8 months, 3 children from 1 family in northern Vietnam died from melioidosis. Burkholderia pseudomallei of the same sequence type, 541, was isolated from clinical samples, borehole water, and garden and rice field soil. Boreholes should be properly constructed and maintained to avoid B. pseudomallei contamination.
The gram-negative soil-dwelling saprophytic bacterium Burkholderia pseudomallei causes melioidosis, a fatal disease highly endemic to Southeast Asia and northern Australia (1). Humans can be infected with B. pseudomallei via inoculation, inhalation, and ingestion. Rice farmers are at high risk for infection because of their frequent exposure to soil and water, but newborns, children, and older persons also are at risk (2,3). We report 3 melioidosis deaths among children in northern Vietnam.
In November 2019, the Preventive Health Center of Soc Son district in Vietnam reported the deaths of 3 children from 1 family. The first child, a 7-year-old girl, had a high fever and abdominal pain on April 6, 2019. Two days later, she was admitted to a local hospital; after 1 day, she was transferred to St. Paul Hospital in Hanoi, where septic shock was diagnosed. She died on April 9, shortly after admission, before any diagnostic tests were performed.
On October 27, 2019, the second child, a 5-year-old boy, had a high fever and abdominal pain around the umbilicus. He was admitted to Vietnam National Children’s Hospital in Hanoi on October 28 with diagnosed septic shock. Abdominal and chest radiographs and abdominal ultrasound results were unremarkable. His blood culture grew B. pseudomallei, and he died on October 31.
The third child, a 13-month-old boy, had a high fever and poor appetite on November 10, 2019. According to his grandparents, he had black stool, like his sister and brother. He was admitted to Vietnam National Children’s Hospital; chest radiography results were unremarkable, but B. pseudomallei was cultured from his blood sample. He died on November 16.
We retrieved laboratory findings from all hospitals to which these children were admitted. Results showed leukopenia, neutropenia, thrombocytopenia, and high procalcitonin and C-reactive protein in all children’s blood. Liver dysfunction was diagnosed in all 3 children, but kidney dysfunction was recognized only in the 2 older children. We detected no identifiable risk factors (Table 1).
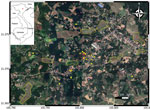
To trace the source of infection, on November 17, 2019, we visited the family home in the midland region of northern Vietnam (Figure 1). During our active surveillance for melioidosis cases admitted to provincial and tertiary hospitals surrounding Hanoi (4), no previous cases had been reported from this area.
We interviewed the parents and grandparents using epidemiologic questions about all the children’s daily activities inside and outside the house. The family used water supplied from 3 boreholes: 1 for bathing (borehole A), 1 for livestock (borehole B), and 1 for human consumption (borehole C). During our first environmental investigation, we collected samples of front garden soil (n = 7), borehole water (n = 9), and boiled drinking water (n = 1). We performed qualitative culture for B. pseudomallei, and all 3 water samples collected from borehole A tested positive (Appendix).
We revisited the home on November 23, 2019, and asked the family about the history of borehole A. In brief, the borehole was drilled in 2010. In 2015, the family reconstructed the back garden and added a new soil layer, resulting in the bore cap being ≈80 cm below the soil surface (Figure 2, panel A). At the end of 2018, the foot valve in the suction pipe of the dynamic electric pump was damaged, and the bore cap was not sealed after the damage was repaired (Figure 2, panel B). We suspected rainwater and surface soil particles contaminated with B. pseudomallei drained into the groundwater via the opened borehole. To test this hypothesis, we conducted a second round of environmental sampling, focusing on borehole A and the nearby surface soil. We collected 26 borehole water and 46 garden soil samples. Within a 1-km radius of the home, we also collected 39 water samples from other boreholes, 30 surface water samples from 10 ponds, and 40 soil samples from 8 rice fields (Figure 1; Appendix).
We found 26 (100%) water samples collected from borehole A and 27 (58.7%) garden soil samples from 8 (80%) sampling points near the borehole were B. pseudomallei–positive by qualitative culture. These findings supported our hypothesis that B. pseudomallei from surface soil might have contaminated the groundwater through the unsealed bore cap during the rainy season, which starts in April and coincided with the first child’s illness and death. Another 5 (12.5%) soil samples from 2 (25%) rice fields also tested B. pseudomallei–positive. Quantitative culture showed that the median B. pseudomallei count was 406 CFU/g (range 12–746 CFU/g) in soil (Appendix). Of 26 water samples collected from borehole A, 2 (7.7%) grew B. pseudomallei on the initial agar plates and had a median B. pseudomallei count of 2 CFU/mL (Table 2).
We selected 20 B. pseudomallei isolates for multilocus sequence typing (MLST) (5): 7 from borehole A, 6 from back garden soil, 5 from rice field soil, and 2 from blood samples from cases 2 and 3. MLST showed an identical sequence type (ST), 541, among all samples (Table 2).
B. pseudomallei is ubiquitously distributed in soil and surface water throughout the tropics, including in Asia, the Pacific Islands, sub-Saharan Africa, and Latin America, where boreholes are the most common water supply in the rural areas (1,6,7). In addition to other waterborne infections (7), untreated water supplies have been implicated in previous human B. pseudomallei infections (8–10). B. pseudomallei also was isolated from the compacted earth floor under the bathing tub of a woman who died from septicemic melioidosis in Brazil (11).
Studies in Australia and Thailand detected diverse STs among B. pseudomallei isolates from an unchlorinated bore water site and a single soil sample (12,13), but our analysis revealed a single ST in the borehole, nearby garden, and surrounding rice fields. Because all 3 infections occurred in children, we believe B. pseudomallei transmission likely occurred through ingestion of contaminated water during bathing, especially considering that the 13-month-old boy was not in contact with garden or rice field soil. Ingestion also could explain the gastrointestinal symptoms the children exhibited.
B. pseudomallei ST541 has been reported from human melioidosis cases in northern Vietnam (3) and has only been described from southeast Asia thus far. During previous surveillance (4), we found other ST541 isolates in clinical and environmental samples from north and north-central Vietnam. An ST541 isolate available in a public MLST database (https://pubmlst.org/organisms/burkholderia-pseudomallei; accessed 2021 Dec 8) was from a human case in Hainan, China, which is close to the area of Vietnam where these 3 melioidosis deaths occurred. From our clinical data retrieval (3,4), 5 of 8 patients infected with B. pseudomallei ST541 died, which could mean ST541 is more virulent than other STs, but further data are needed.
From the epidemiologic investigation and field study at the family home, we became aware of the construction and maintenance of the borehole, which had an unsealed cap and an open borehole below the soil surface. The unsealed borehole probably enabled B. pseudomallei from surface soil to contaminate groundwater during rainfall. Other studies have reported higher rates of gastrointestinal pathogens in water from boreholes with unsealed annuli (14,15). Therefore, persons using boreholes in countries where melioidosis is endemic should ensure proper construction and maintenance to avoid contamination with B. pseudomallei and other pathogens from surface soil.
Mrs. Tran is a PhD candidate at the Laboratory for Microbial Pathogens, VNU–Institute of Microbiology and Biotechnology, Vietnam National University, Hanoi, Vietnam. Her research interests include melioidosis diagnosis and detection of B. pseudomallei from environmental samples.
Acknowledgments
We express our deepest condolences to the family members and sincerely thank them for providing information during the investigation.
This work was supported by Vietnam National University, Hanoi, Vietnam (grant no. QG.21.57). Q.T.L.T. received a PhD scholarship from the Vingroup Innovation Foundation (award no. 2020.TS.33).
References
- Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. DOIGoogle Scholar
- Limmathurotsakul D, Kanoksil M, Wuthiekanun V, Kitphati R, deStavola B, Day NP, et al. Activities of daily living associated with acquisition of melioidosis in northeast Thailand: a matched case-control study. PLoS Negl Trop Dis. 2013;7:
e2072 . DOIPubMedGoogle Scholar - Phuong DM, Trung TT, Breitbach K, Tuan NQ, Nübel U, Flunker G, et al. Clinical and microbiological features of melioidosis in northern Vietnam. Trans R Soc Trop Med Hyg. 2008;102(Suppl 1):S30–6. DOIPubMedGoogle Scholar
- Trinh TT, Nguyen LDN, Nguyen TV, Tran CX, Le AV, Nguyen HV, et al. Melioidosis in Vietnam: recently improved recognition but still an uncertain disease burden after almost a century of reporting. Trop Med Infect Dis. 2018;3:39. DOIPubMedGoogle Scholar
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–79. DOIPubMedGoogle Scholar
- Foster T, Priadi C, Kotra KK, Odagiri M, Rand EC, Willetts J. Self-supplied drinking water in low- and middle-income countries in the Asia-Pacific. npj Clean Water. 2021;4:37.
- Odiyo JO, Mathoni MM, Makungo R. Health risks and potential sources of contamination of groundwater used by public schools in Vhuronga 1, Limpopo Province, South Africa. Int J Environ Res Public Health. 2020;17:6912. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Wongsuvan G, Aanensen D, Ngamwilai S, Saiprom N, Rongkard P, et al. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg Infect Dis. 2014;20:265–8. DOIPubMedGoogle Scholar
- Inglis TJ, Garrow SC, Henderson M, Clair A, Sampson J, O’Reilly L, et al. Burkholderia pseudomallei traced to water treatment plant in Australia. Emerg Infect Dis. 2000;6:56–9.PubMedGoogle Scholar
- Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, Kemp DJ. A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Am J Trop Med Hyg. 2001;65:177–9. DOIPubMedGoogle Scholar
- Rolim DB, Vilar DC, Sousa AQ, Miralles IS, de Oliveira DC, Harnett G, et al. Melioidosis, northeastern Brazil. Emerg Infect Dis. 2005;11:1458–60. DOIPubMedGoogle Scholar
- Mayo M, Kaesti M, Harrington G, Cheng AC, Ward L, Karp D, et al. Burkholderia pseudomallei in unchlorinated domestic bore water, Tropical Northern Australia. Emerg Infect Dis. 2011;17:1283–5. DOIPubMedGoogle Scholar
- Wuthiekanun V, Limmathurotsakul D, Chantratita N, Feil EJ, Day NP, Peacock SJ. Burkholderia Pseudomallei is genetically diverse in agricultural land in Northeast Thailand. PLoS Negl Trop Dis. 2009;3:
e496 . DOIPubMedGoogle Scholar - Knappett PS, McKay LD, Layton A, Williams DE, Alam MJ, Mailloux BJ, et al. Unsealed tubewells lead to increased fecal contamination of drinking water. J Water Health. 2012;10:565–78. DOIPubMedGoogle Scholar
- MacDonald AM, Calow RC. Developing groundwater for secure rural water supplies in Africa. Desalination. 2009;248:546–56. DOIGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 13, 2022
Table of Contents – Volume 28, Number 8—August 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Trung T. Trinh, Laboratory for Microbial Pathogens, VNU–Institute of Microbiology and Biotechnology, Vietnam National University, 144 Xuan Thuy, Cau Giay, Hanoi, Vietnam
Top