Volume 29, Number 2—February 2023
Research
Penicillin and Cefotaxime Resistance of Quinolone-Resistant Neisseria meningitidis Clonal Complex 4821, Shanghai, China, 1965–2020
Figure 3
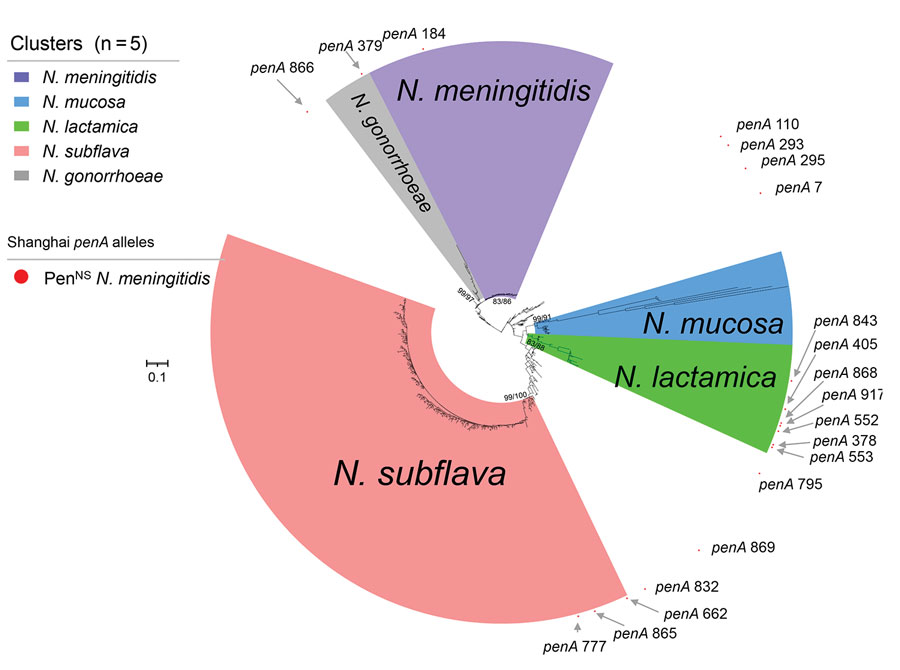
Figure 3. Phylogenetic analysis of penA alleles of Neisseria isolates and genomes, Shanghai, China, 1965–2020, and reference isolates. Phylogenetic analysis of the nucleotide sequences of 580 penA alleles (nucleotides 1321–1722) from N. meningitidis (n = 21,582), N. gonorrhoeae (n = 7,605), N. lactamica (n = 683), N. subflava (n = 431), N. cinerea (n = 65) , N. polysaccharea (n = 52), N. mucosa (n = 33), and other commensal Neisseria (n = 73) isolates and genomes collected in this study and from the Neisseria PubMLST database was constructed by using IQ-TREE version 2.2.0 (23), with both SH-aLRT test and UFboot set as 1,000. The values of SH-aLRT and ultrafast bootstrap (Ufboot) are shown on the node of each clade as SH-aLRT/Ufboot. Clusters were determined by using SH-aLRT values of 80% from the SH-aLRT tests with 1,000 replicates and ultrafast bootstrap (UFBoot) values of 85% from bootstrap tests with 1,000 replicates (IQ-TREE). Alleles penA378, penA405, penA552, penA553, penA843, penA868, and penA917 were within in the N. lactamica cluster; penA662, penA777, and penA865 were within the N. subflava cluster; penA379 was within the N. gonorrhoeae cluster; and the other 8 penA alleles were located outside the 5 clusters. Scale bar indicates substitutions per site. PenNS, penicillin-nonsusceptible meningococci.
References
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J. 2004;23(Suppl):S274–9. DOIPubMedGoogle Scholar
- Maiden MC, Bygraves JA, Feil E, Morelli G, Russell JE, Urwin R, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95:3140–5. DOIPubMedGoogle Scholar
- Shao Z, Li W, Ren J, Liang X, Xu L, Diao B, et al. Identification of a new Neisseria meningitidis serogroup C clone from Anhui province, China. Lancet. 2006;367:419–23. DOIPubMedGoogle Scholar
- Zhou H, Shan X, Sun X, Xu L, Gao Y, Li M, et al. Clonal characteristics of invasive Neisseria meningitidis following initiation of an A + C vaccination program in China, 2005-2012. J Infect. 2015;70:37–43. DOIPubMedGoogle Scholar
- Chen M, Guo Q, Wang Y, Zou Y, Wang G, Zhang X, et al. Shifts in the antibiotic susceptibility, serogroups, and clonal complexes of Neisseria meningitidis in Shanghai, China: a time trend analysis of the pre-quinolone and quinolone eras. PLoS Med. 2015;12:e1001838, discussion e1001838. DOIPubMedGoogle Scholar
- Lucidarme J, Zhu B, Xu L, Bai X, Gao Y, González-López JJ, et al. Genomic analysis of the meningococcal ST-4821 complex-Western clade, potential sexual transmission and predicted antibiotic susceptibility and vaccine coverage. PLoS One. 2020;15:
e0243426 . DOIPubMedGoogle Scholar - Chen M, Harrison OB, Bratcher HB, Bo Z, Jolley KA, Rodrigues CMC, et al. Evolution of sequence type 4821 clonal complex hyperinvasive and quinolone-resistant meningococci. Emerg Infect Dis. 2021;27:1110–22. DOIPubMedGoogle Scholar
- Harrison OB, Cole K, Peters J, Cresswell F, Dean G, Eyre DW, et al. Genomic analysis of urogenital and rectal Neisseria meningitidis isolates reveals encapsulated hyperinvasive meningococci and coincident multidrug-resistant gonococci. Sex Transm Infect. 2017;93:445–51. DOIPubMedGoogle Scholar
- Nadel S, Kroll JS. Diagnosis and management of meningococcal disease: the need for centralized care. FEMS Microbiol Rev. 2007;31:71–83. DOIPubMedGoogle Scholar
- Bozio CH, Isenhour C, McNamara LA. Characteristics of and meningococcal disease prevention strategies for commercially insured persons receiving eculizumab in the United States. PLoS One. 2020;15:
e0241989 . DOIPubMedGoogle Scholar - Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001;344:1378–88. DOIPubMedGoogle Scholar
- Willerton L, Lucidarme J, Walker A, Lekshmi A, Clark SA, Walsh L, et al. Antibiotic resistance among invasive Neisseria meningitidis isolates in England, Wales and Northern Ireland (2010/11 to 2018/19). PLoS One. 2021;16:
e0260677 . DOIPubMedGoogle Scholar - Willerton L, Lucidarme J, Walker A, Lekshmi A, Clark SA, Gray SJ, et al. Increase in penicillin-resistant invasive meningococcal serogroup W ST-11 complex isolates in England. Vaccine. 2021;39:2719–29. DOIPubMedGoogle Scholar
- Taha MK, Vázquez JA, Hong E, Bennett DE, Bertrand S, Bukovski S, et al. Target gene sequencing to characterize the penicillin G susceptibility of Neisseria meningitidis. Antimicrob Agents Chemother. 2007;51:2784–92. DOIPubMedGoogle Scholar
- Xu L, Zhu B, Xu Z, Gao Y, Shao Z. Analysis on antibiotic susceptibility of Neisseria meningitidis isolates in China, 2003–2012 [in Chinese]. Dis Surveill. 2015;30:316–20.
- Xu L, Han FY, Wu D, Zhu BQ, Gao WY, Gao Y, et al. [Analysis on antimicrobial sensitivity of Neisseria meningitidis in China from 2005 to 2019] [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi. 2021;55:207–11.PubMedGoogle Scholar
- Zhang Y, Deng X, Jiang Y, Zhang J, Zhan L, Mei L, et al. The epidemiology of meningococcal disease and carriage, genotypic characteristics and antibiotic resistance of Neisseria meningitidis isolates in Zhejiang Province, China, 2011–2021. Front Microbiol. 2022;12:
801196 . DOIPubMedGoogle Scholar - Chen M, Zhang C, Zhang X, Chen M. Meningococcal quinolone resistance originated from several commensal Neisseria species. Antimicrob Agents Chemother. 2020;64:e01494–19. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 32nd ed. Supplement M100. Wayne (PA): The Institute; 2022.
- Bratcher HB, Corton C, Jolley KA, Parkhill J, Maiden MC. A gene-by-gene population genomics platform: de novo assembly, annotation and genealogical analysis of 108 representative Neisseria meningitidis genomes. BMC Genomics. 2014;15:1138. DOIPubMedGoogle Scholar
- Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. DOIPubMedGoogle Scholar
- Demczuk W, Sidhu S, Unemo M, Whiley DM, Allen VG, Dillon JR, et al. Neisseria gonorrhoeae sequence typing for antimicrobial resistance, a novel antimicrobial resistance multilocus typing scheme for tracking global dissemination of N. gonorrhoeae strains. J Clin Microbiol. 2017;55:1454–68. DOIPubMedGoogle Scholar
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74. DOIPubMedGoogle Scholar
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1:
vev003 . DOIPubMedGoogle Scholar - Lee K, Nakayama SI, Osawa K, Yoshida H, Arakawa S, Furubayashi KI, et al. Clonal expansion and spread of the ceftriaxone-resistant Neisseria gonorrhoeae strain FC428, identified in Japan in 2015, and closely related isolates. J Antimicrob Chemother. 2019;74:1812–9. DOIPubMedGoogle Scholar
- Van Esso D, Fontanals D, Uriz S, Morera MA, Juncosa T, Latorre C, et al. Neisseria meningitidis strains with decreased susceptibility to penicillin. Pediatr Infect Dis J. 1987;6:438–9. DOIPubMedGoogle Scholar
- Richter SS, Gordon KA, Rhomberg PR, Pfaller MA, Jones RN. Neisseria meningitidis with decreased susceptibility to penicillin: report from the SENTRY antimicrobial surveillance program, North America, 1998-99. Diagn Microbiol Infect Dis. 2001;41:83–8. DOIPubMedGoogle Scholar
- Bijlsma MW, Bekker V, Brouwer MC, Spanjaard L, van de Beek D, van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960-2012: an analysis of national surveillance data. Lancet Infect Dis. 2014;14:805–12. DOIPubMedGoogle Scholar
- Lahra MM, George CRR, Shoushtari M, Hogan TR. Australian Meningococcal Surveillance Programme Annual Report, 2020. Commun Dis Intell (2018). 2021;45:45. DOIPubMedGoogle Scholar
- Deghmane AE, Hong E, Taha MK. Emergence of meningococci with reduced susceptibility to third-generation cephalosporins. J Antimicrob Chemother. 2017;72:95–8. DOIPubMedGoogle Scholar
- Tomberg J, Unemo M, Davies C, Nicholas RA. Molecular and structural analysis of mosaic variants of penicillin-binding protein 2 conferring decreased susceptibility to expanded-spectrum cephalosporins in Neisseria gonorrhoeae: role of epistatic mutations. Biochemistry. 2010;49:8062–70. DOIPubMedGoogle Scholar
- Nakayama S, Shimuta K, Furubayashi K, Kawahata T, Unemo M, Ohnishi M. New ceftriaxone- and multidrug-resistant Neisseria gonorrhoeae strain with a novel mosaic penA gene isolated in Japan. Antimicrob Agents Chemother. 2016;60:4339–41. DOIPubMedGoogle Scholar
- Li J, Li Y, Shao Z, Li L, Yin Z, Ning G, et al. Prevalence of meningococcal meningitis in China from 2005 to 2010. Vaccine. 2015;33:1092–7. DOIPubMedGoogle Scholar
- Mowlaboccus S, Jolley KA, Bray JE, Pang S, Lee YT, Bew JD, et al. Clonal expansion of new penicillin-resistant clade of Neisseria meningitidis serogroup W clonal complex 11, Australia. Emerg Infect Dis. 2017;23:1364–7. DOIPubMedGoogle Scholar
- Potts CC, Retchless AC, McNamara LA, Marasini D, Reese N, Swint S, et al.; Antimicrobial-Resistant Neisseria meningitidis Team. Antimicrobial-Resistant Neisseria meningitidis Team. Acquisition of ciprofloxacin resistance among an expanding clade of beta-lactamase positive, serogroup Y Neisseria meningitidis in the United States. Clin Infect Dis. 2021;73:1185–93. DOIPubMedGoogle Scholar
- Marín JEO, Villatoro E, Luna MJ, Barrientos AM, Mendoza E, Lemos APS, et al. Emergence of MDR invasive Neisseria meningitidis in El Salvador, 2017-19. J Antimicrob Chemother. 2021;76:1155–9. DOIPubMedGoogle Scholar
- Chinese Preventive Medicine Association. [Experts’ consensus on immunization with meningococcal vaccines in China] [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2019;40:123–8.PubMedGoogle Scholar
- Xu J, Chen Y, Yue M, Yu J, Han F, Xu L, et al. Prevalence of Neisseria meningitidis serogroups in invasive meningococcal disease in China, 2010 - 2020: a systematic review and meta-analysis. Hum Vaccin Immunother. 2022;18:
2071077 . DOIPubMedGoogle Scholar - Li J, Shao Z, Liu G, Bai X, Borrow R, Chen M, et al. Meningococcal disease and control in China: Findings and updates from the Global Meningococcal Initiative (GMI). J Infect. 2018;76:429–37. DOIPubMedGoogle Scholar
- Chen M, Rodrigues CMC, Harrison OB, Zhang C, Tan T, Chen J, et al. Invasive meningococcal disease in Shanghai, China from 1950 to 2016: implications for serogroup B vaccine implementation. Sci Rep. 2018;8:12334. DOIPubMedGoogle Scholar
1These authors contributed equally to this article.