Volume 29, Number 4—April 2023
Synopsis
Association of Scrub Typhus in Children with Acute Encephalitis Syndrome and Meningoencephalitis, Southern India
Abstract
Scrub typhus is an established cause of acute encephalitis syndrome (AES) in northern states of India. We systematically investigated 376 children with AES in southern India, using a stepwise diagnostic strategy for the causative agent of scrub typhus, Orientia tsutsugamushi, including IgM and PCR testing of blood and cerebrospinal fluid (CSF) to grade its association with AES. We diagnosed scrub typhus in 87 (23%) children; of those, association with AES was confirmed in 16 (18%) cases, probable in 55 (63%), and possible in 16 (18%). IgM detection in CSF had a sensitivity of 93% and specificity of 82% compared with PCR. Our findings suggest scrub typhus as an emerging common treatable cause of AES in children in southern India and highlight the importance of routine testing for scrub typhus in diagnostic algorithms. Our results also suggest the potential promise of IgM screening of CSF for diagnosis of AES resulting from scrub typhus.
Scrub typhus is an acute febrile illness caused by an obligate intracellular gram-negative bacterium, Orientia tsutsugamushi. It is transmitted through chigger mites and is considered endemic to the tsutsugamushi triangle (covering Asia, northern Australia, and islands in the Indian and Pacific Oceans), although scrub typhus caused by other Orientia species has also been reported in Africa, France, the Middle East, and South America (1). A recent systematic review from hospital-based studies in India reported 25% of acute undifferentiated febrile illness was caused by scrub typhus. Most studies included were from southern India, but only 20% of included patients were <15 years of age (2). Although scrub typhus illness is typically self-limiting, neurologic complications are seen in 20%–25% of patients admitted to the hospital and are associated with high mortality rates (3,4). Scrub typhus can result in myriad neurologic manifestations, including meningitis, meningoencephalitis, encephalopathy, seizures, stroke, neuropathy, optic neuritis, myositis, myelitis, involuntary movements, and Guillain-Barré syndrome, all of which are well recognized in adults (3,4).
Recent studies in India have identified O. tsutsugamushi as a major cause of acute encephalitis syndrome (AES) outbreaks, especially in northern states of the country, such as Uttar Pradesh, Bihar, West Bengal, and Assam (5–7). Outbreaks of AES pose a major public health problem in India, predominantly affecting children (8). The definition of AES used for syndromic surveillance is broad and includes all patients experiencing acute onset of fever and altered mental state (9,10). The clinical manifestation might be caused by encephalitis or meningitis (direct invasion of the central nervous system [CNS] by the pathogen) or encephalopathy without CNS invasion, such as in the case of severe systemic infection, metabolic derangement, or other neurologic complications after the infection (10,11). Identifying the pathogenesis could inform management and prognosis (10,12).
Early diagnosis is key to initiating prompt specific treatment, which can reduce complications and fatality rates of scrub typhus (2,13). Clinical diagnosis can be challenging because of the overlap of symptoms with other tropical infections endemic to the area that can also cause AES (5), such as dengue, chikungunya, malaria, and leptospirosis (14). Current microbiological diagnostics for scrub typhus, which are usually based on detecting IgM in serum samples or nucleic acid by PCR, have limitations. IgM appears in serum 5–6 days after onset of illness, can persist long after acute illness, and might cross-react with IgM of other cocirculating pathogens (14,15). Therefore, in AES patients with simultaneous microbiological evidence for another potential pathogen and O. tsutsugamushi, confirming O. tsutsugamushi as the cause is difficult. Detection of IgM in cerebrospinal fluid (CSF) is yet to be used widely in patients with suspected neurologic scrub typhus. Immunofluorescence assay has long been considered the reference standard serologic test, but its use is limited by expense and challenges in interpretation. PCR might help overcome shortcomings of serologic tests with respect to cross-reacting and persisting antibodies, but a positive result is only likely during the bacteremia phase of infection (16). Moreover, the recommended samples for O. tsutsugamushi PCR are blood or eschar material, whereas the sensitivity of PCR on CSF remains unclear (7,16,17). Therefore, a diagnostic approach using accessible tests to determine the association of scrub typhus with AES is urgently needed.
We present preliminary findings of an ongoing multicenter prospective cohort study suggesting scrub typhus as a cause of AES in children in southern India. We used a diagnostic strategy to investigate the association of scrub typhus with AES. We describe the clinical spectrum, epidemiology, and laboratory findings of children with scrub typhus manifesting as AES. We then identify patients demonstrating evidence of meningoencephalitis or encephalitis and explore the value of performing IgM ELISA on CSF samples.
Patients and Study Sites
We prospectively enrolled pediatric patients from 1 month to 18 years of age who fulfilled the Indian National Vector Borne Disease Control Programme (NVBDCP) and World Health Organization case definition of AES (8) (Appendix Table 1) and with illness duration of <30 days at the time of hospital admission. Patients were those treated at 3 tertiary-care hospitals in Bangalore, Karnataka state, India (Indira Gandhi Institute of Child Health, St. John’s Medical College and Hospital, and Vani Vilas Hospital), during March 2019–March 2022.
Ethics Statement
The study was approved by the institutional ethics and review boards of the hospitals and the coordinating center, National Institute of Mental Health and Neurosciences. Full informed consent was taken by the study team, who were trained specifically in taking consent from caregivers, and assent from older children, using procedures and forms approved by the institutional ethics committees.
Clinical Assessment and Data Collection
Clinical coinvestigators (V.K.G., L.A.V., F.S.D., S.S., M.K.) from the 3 centers performed clinical and neurologic examination of patients. After obtaining consent, we entered detailed clinical history and examination findings on an electronic clinical proforma. Results of routine laboratory tests and patient demographics were collected and entered online by N.P., S.M., or T.D. We determined the normal range of routine laboratory tests according to the age of the patient (18) and defined single-organ dysfunction and multiorgan dysfunction syndrome according to established criteria (19).
Microbiological Testing
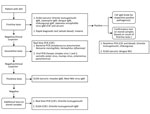
Blood and CSF specimens of enrolled patients were tested at the Department of Neurovirology, National Institute of Mental Health and Neurosciences, by using a laboratory algorithm designed by Ravi et al. (5) with some modifications (Figure 1). First-line tests included serum IgM ELISA for various pathogens. CSF samples of patients with IgM-positive ELISA serum samples were diluted in 1:10 proportion for detection of IgM. We performed confirmatory tests on IgM-positive patients, including real-time PCR for O. tsutsugamushi on CSF and blood samples. For PCR, we extracted DNA from samples by using the QIAamp DNA mini kit (QIAGEN, https://www.qiagen.com) and performed real-time PCR targeting the 47kDa protein gene using the protocol described by Jiang et al. (20). In addition, we also performed real-time PCR and IgM ELISA for O. tsutsugamushi on stored CSF samples of patients with a negative result after third-line tests. We used the Scrub Typhus Detect IgM ELISA kit (InBios International, http://inbios.com) and considered an optical density (OD) cutoff of 0.8 in serum (15) and 0.5 in CSF (21) samples to be positive. Scrub typhus was diagnosed in patients with IgM-positive real-time PCR or ELISA.
The level of certainty of association of scrub typhus with AES in cases positive for >1 microbiological test(s) for O. tsutsugamushi was determined by using criteria determined by Granerod et al. (11) with modifications (Tables 1, 2). We identified patients with meningoencephalitis/encephalitis (ME) and scrub typhus ME as those demonstrating clinical signs of either encephalitis or meningoencephalitis (Table 2).
Statistical Analysis
We performed statistical analysis by using R version 3.6.3 (The R Project for Statistical Computing, https://www.r-project.org). We presented descriptive data for categorical variables as frequencies, percentages, or both and described continuous variables using mean +SD or median and interquartile range (IQR). To describe the diagnostic accuracy of CSF IgM, we compared results against CSF PCR to calculate the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of CSF IgM with 95% CI. We also calculated those values for patients with scrub typhus ME.
We included a total of 376 children with AES in the study (Appendix Figure 1). Of those, scrub typhus was diagnosed in 87 patients by using the laboratory algorithm described.
Microbiological Testing
We collected samples for microbiological testing a median of 11 (IQR 8–14) days from onset of symptoms and median of 4 (IQR 2–6) days after hospitalization. Serum samples were positive for O. tsutsugamushi IgM in 86/376 (22.8%) patients. Of those 86 patients, 39 (45.4%) had a positive microbiological test result for another pathogen (referred to as copositive) (Appendix Table 2); 47 (54.6%) were positive for O. tsutsugamushi IgM alone (referred to as single-positive).
CSF samples were available for 82/86 patients with O. tsutsugamushi IgM–positive serum samples and all 184 patients who had no etiologic diagnosis after use of the laboratory algorithm. CSF samples were IgM-positive in 58/82 (71%) patients (23/36 of copositive patients and 35/45 of single-positive patients). All 184 serum IgM-seronegative patients were negative for CSF IgM by ELISA.
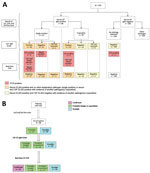
Real-time PCR results were positive in 15/86 (17%) patients with IgM-positive serum (real-time PCR of both CSF and blood was positive in 2 patients; 11 were positive by CSF PCR only and 2 by blood PCR only). Of the 184 CSF samples of patients with no etiologic diagnosis after first-line and second-line tests, 1 was positive by real-time PCR for O. tsutsugamushi. In total, 16 patients were positive for O. tsutsugamushi by PCR. Therefore, of 376 patients with AES, 87 (23%) had a positive microbiological test for scrub typhus (AES–scrub typhus) (Figure 2).
Diagnostic Association of Scrub Typhus with AES
On the basis of serum IgM results, the association of scrub typhus with AES was probable in 47/87 (54%) patients and possible in 39/87 (45%) patients. Further, on performing IgM ELISA on CSF samples, the association was probable (single-positive) in 47 (58.8%) persons, probable (copositive) in 23 (26.4%) persons, and possible in 16 (18%) persons. Finally, on the basis of real-time PCR results, the association was confirmed in 16 (18%) patients, probable (single-positive) in 38 (43.7%) patients, probable (copositive) in 17 (19.5%) patients, and possible in 16 (18.4%) patients (Figure 2).
ME and Scrub Typhus ME
Of the 87 patients, 65 (74.7%) had findings suggestive of ME (Appendix Tables 3, 4). The diagnostic association of ME with scrub typhus was confirmed or probable (single-positive) in 54 (62%) patients (Figure 2), and of those patients, 43 had ME. Therefore, among all 87 patients, 49.4% had scrub typhus ME (Figure 3).
Diagnostic Accuracy of CSF IgM Testing
We performed IgM ELISA and real-time PCR on CSF samples of 266 patients (i.e., 82/86 patients with IgM-positive serum samples and 184/184 patients with no etiology after tests were performed per the laboratory algorithm). We created a 2×2 table to compare the performance of CSF IgM with CSF PCR. The sensitivity of CSF IgM ELISA was 92.9% (95% CI 66.1%–99.8%), specificity 82.1% (95% CI 76.8%–86.6%), PPV 22.4% (12.5%–35.2%), and NPV 99.5% (97.3%–100%) (Table 3).
CSF samples were available for 53/54 patients with confirmed or probable (single-positive) scrub typhus. CSF IgM was positive in 36/42 (85.7%) patients with ME and 5/11 (45.5%) patients without ME. Sensitivity of CSF IgM in patients with ME was 85.7% (95% CI 71.4–94.5%) and specificity was 54.5% (95% CI 23.3%–83.2%); the corresponding PPV was 87.8% (78.8%–93.3%) and NPV was 50.0% (28.6%–71.4%) (Figure 3).
Demographic and Clinical Profile
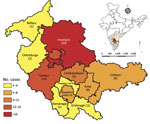
The male:female ratio of children with scrub typhus was 1.5:1. Ages ranged from 2 months to 17 years; the mean age was 8.5 (SD +4) years (Table 4). Proportions of AES-scrub typhus cases were highest in the months of August and September. In addition, the number of AES-scrub typhus patients and their proportion of total AES patients followed the same pattern as the total number of AES cases (Appendix Figures 2, 3). The largest percentage of children (37%) were from Anantapur district in Andhra Pradesh state, followed by 17% from Tumkur district in Karnataka state (Figure 4). Nearly 48% of patients were referred from another hospital, and 34% received anti-infective medications before being admitted to the study hospital. The median duration of illness before admission to the study hospital was 6 (IQR 4–9.5) days.
All 87 children experienced fever and change in mental state; fever was the first symptom in 95% of cases. Around 62% of children had seizures; generalized tonic-clonic seizures were the most common type (74%), and some patients also had focal, tonic, or absence seizures. Upon examination at the time of hospital admission, 55 (64%) patients had altered mental state. The Glasgow Coma Scale at admission ranged from 3 to 15; the median was 13 (IQR 10–15) (Table 5). Signs of meningeal irritation were detected in 48% of patients, cerebellar signs in 21%, and papilledema in 20%. Other neurologic findings were cranial nerve abnormalities (6%), involuntary movements (9%) and photophobia (9%), abnormal tone (50%), decreased power (19%), and abnormal plantar reflexes (24%) (Table 5). Approximately 39% of the patients met criteria for multiorgan dysfunction syndrome (Appendix Table 5).
Laboratory Findings
Anemia, leukocytosis, thrombocytopenia, transaminitis, hypoalbuminemia, and uremia were each present in >50% of patients (Table 6). CSF results revealed lymphocytic pleocytosis and elevated protein concentration in most patients (Appendix Table 6).
Treatment
Of the patients with scrub typhus, 44 (51%) required care in the intensive care unit during their hospitalization, and 26 of those required ventilatory support. All patients except 1 were prescribed doxycycline (100 mg 2×/d for 10 days). One patient died during hospitalization.
Our findings suggest that scrub typhus is a major cause of AES in children in southern India. Of 193 (51%) patients with a known etiology, a microbiological test for O. tsutsugamushi was positive in 87 (45%) patients, making it the most common etiology obtained in the study. An increasing number of studies in Asia have reported the contribution of O. tsutsugamushi to the burden of acute febrile illness in the continent, including South Korea, Japan, China, Taiwan, Thailand, and Bhutan, countries where scrub typhus is a notifiable disease (24). Studies including screening for O. tsutsugamushi as part of systematic surveillance of childhood CNS infections in Cambodia, Vietnam, Laos, Myanmar, and Thailand report its presence in 1%–4.7% of children (17,25–28). Although studies in India have documented meningoencephalitis as a manifestation of scrub typhus in children (2,29,30), our study highlights the importance of systematic screening for scrub typhus in children with AES in southern India. Scrub typhus is a well-recognized cause of acute febrile illness in the major southern Indian states of Andhra Pradesh and Karnataka (31–33), but we report scrub typhus is also a common cause of AES in children from these states.
Given the challenges in clinical diagnosis (10,14,15) and complexity of defining the causal relationship of scrub typhus with AES on the basis of serum IgM ELISA, the most widely used test for scrub typhus (15), we used a causality strategy. This diagnostic strategy helped in differentiating the certainty of association of 87 AES–scrub typhus cases into 16 cases with confirmed association, 55 with probable association, and 16 with possible association. Real-time PCR, which is confirmatory for scrub typhus, was positive in 6/39 (15%) cases with microbiological evidence of another pathogen and increased the diagnostic association from possible to confirmed. We were able to diagnose scrub typhus in 1 extra case in which IgM ELISA for O. tsutsugamushi and tests for other pathogens were negative. Despite systematic testing, the prevalence of positive real-time PCR in children with AES caused by scrub typhus was low in our study (16 [18%] children), although still higher than in other studies (7,34). PCR positivity might be maximized by collecting clinical samples sooner after illness onset and using whole blood or buffy coat instead of serum to capture intracellular bacteria (14,16). In this study, patients with a positive PCR had a median duration of illness of 9 (IQR 5.75–12.25) days before clinical specimen sampling versus 11 (IQR 8.5–14.5) days for patients with a negative PCR result.
Because IgM does not ordinarily cross the blood–CSF barrier, presence of those antibodies in CSF implies their production within the CNS (35) and higher certainty of association with the infection compared to serum IgM. Using CSF IgM ELISA increased the certainty of association from possible to probable in 23 patients who had simultaneous evidence of another pathogen. Although the kit is recommended for detecting IgM in serum samples only, Murhekar et al. (6) observed good correlation between OD values for O. tsutsugamushi IgM in serum and CSF. They determined a cutoff OD value of 0.22 after testing CSF samples from 374 children <14 years of age with AES in Gorakhpur, Uttar Pradesh state, India (35). A cutoff OD value for IgM in CSF has not been determined in the southern states in India, so we used a higher cutoff (0.5), as used by Behera et al. (21) for CSF of children with scrub typhus ME in eastern India.
Our results demonstrate that, compared with PCR, IgM ELISA of CSF had a sensitivity of 92.9%, but with a wide 95% CI, suggesting the estimate is less precise. Although the comparison is indirect, that sensitivity is similar to that of serum IgM by the same ELISA kit (92.4%) used for patients with acute febrile illness caused by scrub typhus in southern India (14). The specificity of CSF IgM ELISA was moderate compared to PCR at 82%. That finding might be because PCR positivity was less common in our study, which could be explained by delayed sampling during the course of illness, resulting in a higher likelihood of detection of IgM than DNA. In addition, the use of a single reference standard (PCR) in our study could result in a low PPV of IgM ELISA of CSF. The sensitivity of CSF IgM in patients with scrub typhus ME was 85.7%. Because only 11 patients did not have features suggestive of ME, ascertaining the true specificity is difficult.
Almost three quarters of the patients with AES-scrub typhus had meningoencephalitis. Distinguishing patients with scrub typhus ME from patients with encephalopathy with other causes is crucial. Therapeutic failure of doxycycline, the drug of choice for scrub typhus, has been reported in patients with scrub typhus ME (36). This failure could be caused by inadequate concentration of doxycycline in CSF at conventional doses and might indicate the need for increased dosages, intravenous administration, or administration of other antimicrobial agents such as rifampin that have good penetration to the CNS. However, the efficacy of this treatment is yet to be proven (37,38).
The neurologic manifestations in children with scrub typhus that meet the broader epidemiologic definition of AES are rarely reported (13,25,39,40), and no data from southern India have been published. Of all children with scrub typhus in our study, 8 (9%) had involuntary hyperkinetic movements that are rare neurologic manifestations of scrub typhus more often reported in adults than children (41). Opsoclonus-myoclonus, best recognized as part of opsoclonus-myoclonus-ataxia syndrome associated with neuroblastoma in children, is rarely caused by infections (13,41). Only 2 such cases of scrub typhus associated with pediatric opsoclonus-myoclonus-ataxia syndrome have been reported from India (42,43). Cerebellar signs, which are uncommon in children with scrub typhus (3,13), were noted in almost one fifth of the children in our study. As reported by Vishwanath et al. (30), the sixth cranial nerve was the most affected cranial nerve. Papilledema was detected in 20% of children in our study. Few studies have reported direct retinal involvement and isolated optic disc edema in the absence of raised intracranial pressure in scrub typhus (29,44,45); however, findings in this area remain inconclusive in our study. Presence of eschar typically occurs in 4%–46% of patients with scrub typhus; therefore, while specific, eschar is not a sensitive marker (30), and it was found in only 5% of patients in this study.
The first limitation of our study is that, whereas serum IgM ELISA is the most widely used specific test for O. tsutsugamushi, we used a single-positive IgM result as a criterion for diagnosis of scrub typhus. Obtaining serial blood samples and performing immunofluorescence or similar assays to demonstrate a 4-fold rise in antibody titers would have enabled more certainty in the diagnosis, especially in cases in which antibodies to another pathogen were detected. However, we defined those patients as having possible scrub typhus to allow for this uncertainty, and they comprised only 18% of the scrub typhus patients in this study. Also, for IgM detection in CSF, we relied on a cutoff value widely used for serum IgM, because a cutoff value for CSF has not been determined in this region. Furthermore, we could not perform sequencing of the PCR-amplified nucleic acid or characterization of surface antigen because of limited resources.
In this study, despite limited accessibility and shortcomings of reference standard tests, we present a stepwise approach to identify scrub typhus as a probable or confirmed etiology by using tests that are relatively easy to access and perform. Our findings highlight the importance of systematic routine testing for the treatable and common pathogen O. tsutsugamushi in all patients with AES in southern India, as is practiced in several states in northern India. This testing could have a notable effect on the approach to clinical management and public health interventions for patients with AES. Apart from reinforcing common clinical, epidemiologic, and laboratory findings reported by other studies (13,29,39,40,46), we report insights into the neurologic spectrum of scrub typhus in children, which appears to be broad and underreported.
Finally, CSF IgM ELISA is a promising test for patients with AES caused by scrub typhus, which requires evaluation in a larger population and determination of a region-specific cutoff OD value. Combining CSF PCR with CSF IgM ELISA wherever feasible might increase the certainty of association between AES and scrub typhus.
Dr. Damodar is an India Alliance DBT/Wellcome Trust early career fellow (Clinical and Public Health) in the Department of Neurovirology, National Institute of Mental Health and Neurosciences (Bangalore, India). Her research interests include strengthening diagnostics for infectious diseases of international public health concern.
Acknowledgments
We thank Manisha Biswal and Jasleen Kaur for providing us with positive control and technical advice to standardize real-time PCR for Orientia tsutsugamushi. We thank Reeta Mani for standardizing bacterial and chikungunya real-time PCR used as part of the laboratory algorithm.
This work was supported by DBT/Wellcome Trust India Alliance Fellowship IA/E/15/1/503960 awarded to T.D. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Jiang J, Richards AL. Scrub typhus: no longer restricted to the tsutsugamushi triangle. Trop Med Infect Dis. 2018;3:11. DOIPubMedGoogle Scholar
- Devasagayam E, Dayanand D, Kundu D, Kamath MS, Kirubakaran R, Varghese GM. The burden of scrub typhus in India: A systematic review. PLoS Negl Trop Dis. 2021;15:
e0009619 . DOIPubMedGoogle Scholar - Garg D, Manesh A. Neurological facets of scrub typhus: a comprehensive narrative review. Ann Indian Acad Neurol. 2021;24:849–64. DOIPubMedGoogle Scholar
- Varghese GM, Trowbridge P, Janardhanan J, Thomas K, Peter JV, Mathews P, et al. Clinical profile and improving mortality trend of scrub typhus in South India. Int J Infect Dis. 2014;23:39–43. DOIPubMedGoogle Scholar
- Ravi V, Hameed SKS, Desai A, Mani RS, Reddy V, Velayudhan A, et al. An algorithmic approach to identifying the aetiology of acute encephalitis syndrome in India: results of a 4-year enhanced surveillance study. Lancet Glob Health. 2022;10:e685–93. DOIPubMedGoogle Scholar
- Murhekar MV, Vivian Thangaraj JW, Sadanandane C, Mittal M, Gupta N, Rose W, et al. Investigations of seasonal outbreaks of acute encephalitis syndrome due to Orientia tsutsugamushi in Gorakhpur region, India: A One Health case study. Indian J Med Res. 2021;153:375–81. DOIPubMedGoogle Scholar
- Jain P, Prakash S, Tripathi PK, Chauhan A, Gupta S, Sharma U, et al. Emergence of Orientia tsutsugamushi as an important cause of Acute Encephalitis Syndrome in India. PLoS Negl Trop Dis. 2018;12:
e0006346 . DOIPubMedGoogle Scholar - Sen PK, Dhariwal AC, Jaiswal RK, Lal S, Raina VK, Rastogi A. Epidemiology of acute encephalitis syndrome in India: changing paradigm and implication for control. J Commun Dis. 2014;46:4–11.
- Government of India. Guidelines for surveillance of acute encephalitis syndrome (with special reference to Japanese encephalitis). November 2006 [cited 2022 Jul 1]. http://nvbdcp.gov.in/WriteReadData/l892s/AES_guidelines.pdf
- John TJ, Verghese VP, Arunkumar G, Gupta N, Swaminathan S. The syndrome of acute encephalitis in children in India: Need for new thinking. Indian J Med Res. 2017;146:158–61. DOIPubMedGoogle Scholar
- Granerod J, Cunningham R, Zuckerman M, Mutton K, Davies NWS, Walsh AL, et al. Causality in acute encephalitis: defining aetiologies. Epidemiol Infect. 2010;138:783–800. DOIPubMedGoogle Scholar
- Venkatesan A, Geocadin RG. Diagnosis and management of acute encephalitis: A practical approach. Neurol Clin Pract. 2014;4:206–15. DOIPubMedGoogle Scholar
- Misra UK, Kalita J, Mani VE. Neurological manifestations of scrub typhus. J Neurol Neurosurg Psychiatry. 2015;86:761–6. DOIPubMedGoogle Scholar
- Kannan K, John R, Kundu D, Dayanand D, Abhilash KPP, Mathuram AJ, et al. Performance of molecular and serologic tests for the diagnosis of scrub typhus. PLoS Negl Trop Dis. 2020;14:
e0008747 . DOIPubMedGoogle Scholar - Varghese GM, Rajagopal VM, Trowbridge P, Purushothaman D, Martin SJ. Kinetics of IgM and IgG antibodies after scrub typhus infection and the clinical implications. Int J Infect Dis. 2018;71:53–5. DOIPubMedGoogle Scholar
- Koh GC, Maude RJ, Paris DH, Newton PN, Blacksell SD. Diagnosis of scrub typhus. Am J Trop Med Hyg. 2010;82:368–70. DOIPubMedGoogle Scholar
- Pommier JD, Gorman C, Crabol Y, Bleakley K, Sothy H, Santy K, et al.; SEAe Consortium. Childhood encephalitis in the Greater Mekong region (the SouthEast Asia Encephalitis Project): a multicentre prospective study. Lancet Glob Health. 2022;10:e989–1002. DOIPubMedGoogle Scholar
- Lo Stanley F. Reference intervals for laboratory tests and procedures. In: Kleigman RM, Behrman RE, Jenson HB, Stanton BP, editors. Nelson textbook of pediatrics. 20th ed. Philadelphia: Saunders Elsevier; 2011. p. 3464–3472.
- Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. DOIPubMedGoogle Scholar
- Jiang J, Chan TC, Temenak JJ, Dasch GA, Ching WM, Richards AL. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–6. DOIPubMedGoogle Scholar
- Behera B, Satapathy AK, Ranjan J, Chandrasekar S, Patel S, Mishra B, et al. Profile of scrub typhus meningitis/meningoencephalitis in children with and without scrub typhus IgM antibody in CSF. J Neurosci Rural Pract. 2021;12:786–91. DOIPubMedGoogle Scholar
- Granerod J, Ambrose HE, Davies NW, Clewley JP, Walsh AL, Morgan D, et al.; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10:835–44. DOIPubMedGoogle Scholar
- Jaijakul S, Salazar L, Wootton SH, Aguilera E, Hasbun R. The clinical significance of neutrophilic pleocytosis in cerebrospinal fluid in patients with viral central nervous system infections. Int J Infect Dis. 2017;59:77–81. DOIPubMedGoogle Scholar
- Bonell A, Lubell Y, Newton PN, Crump JA, Paris DH. Estimating the burden of scrub typhus: A systematic review. PLoS Negl Trop Dis. 2017;11:
e0005838 . DOIPubMedGoogle Scholar - Horwood PF, Duong V, Laurent D, Mey C, Sothy H, Santy K, et al. Aetiology of acute meningoencephalitis in Cambodian children, 2010-2013. Emerg Microbes Infect. 2017;6:
e35 . DOIPubMedGoogle Scholar - Olsen SJ, Campbell AP, Supawat K, Liamsuwan S, Chotpitayasunondh T, Laptikulthum S, et al.; Thailand Encephalitis Surveillance Team. Infectious causes of encephalitis and meningoencephalitis in Thailand, 2003-2005. Emerg Infect Dis. 2015;21:280–9. DOIPubMedGoogle Scholar
- Dittrich S, Rattanavong S, Lee SJ, Panyanivong P, Craig SB, Tulsiani SM, et al. Orientia, rickettsia, and leptospira pathogens as causes of CNS infections in Laos: a prospective study. Lancet Glob Health. 2015;3:e104–12. DOIPubMedGoogle Scholar
- Dubot-Pérès A, Mayxay M, Phetsouvanh R, Lee SJ, Rattanavong S, Vongsouvath M, et al. Management of central nervous system infections, Vientiane, Laos, 2003–2011. Emerg Infect Dis. 2019;25:898–910. DOIPubMedGoogle Scholar
- Dinesh Kumar N, Arun Babu T, Vijayadevagaran V, Ananthakrishnan S, Kittu D. Clinical profile of scrub typhus meningoencephalitis among South Indian children. J Trop Pediatr. 2018;64:472–8.PubMedGoogle Scholar
- Viswanathan S, Muthu V, Iqbal N, Remalayam B, George T. Scrub typhus meningitis in South India—a retrospective study. PLoS One. 2013;8:
e66595 . DOIPubMedGoogle Scholar - Subbalaxmi MV, Madisetty MK, Prasad AK, Teja VD, Swaroopa K, Chandra N, et al. Outbreak of scrub typhus in Andhra Pradesh—experience at a tertiary care hospital. J Assoc Physicians India. 2014;62:490–6.PubMedGoogle Scholar
- Usha K, Kumar E, Kalawat U, Kumar BS, Chaudhury A, Gopal DV. Molecular characterization of Orientia tsutsugamushi serotypes causing scrub typhus outbreak in southern region of Andhra Pradesh, India. Indian J Med Res. 2016;144:597–603.PubMedGoogle Scholar
- Mørch K, Manoharan A, Chandy S, Chacko N, Alvarez-Uria G, Patil S, et al. Acute undifferentiated fever in India: a multicentre study of aetiology and diagnostic accuracy. BMC Infect Dis. 2017;17:665. DOIPubMedGoogle Scholar
- Khan SA, Bora T, Laskar B, Khan AM, Dutta P. Scrub typhus leading to acute encephalitis syndrome, Assam, India. Emerg Infect Dis. 2017;23:148–50. DOIPubMedGoogle Scholar
- Gupte MD, Gupte M, Kamble S, Mane A, Sane S, Bondre V, et al. Detection of immunoglobulin m and immunoglobulin g antibodies against Orientia tsutsugamushi for scrub typhus diagnosis and serosurvey in endemic regions. Indian Pediatr. 2020;57:1131–4. DOIPubMedGoogle Scholar
- Kim DM, Chung JH, Yun NR, Kim SW, Lee JY, Han MA, et al. Scrub typhus meningitis or meningoencephalitis. Am J Trop Med Hyg. 2013;89:1206–11. DOIPubMedGoogle Scholar
- Peter JV, Sudarsan TI, Prakash JA, Varghese GM. Severe scrub typhus infection: Clinical features, diagnostic challenges and management. World J Crit Care Med. 2015;4:244–50. DOIPubMedGoogle Scholar
- Varghese GM, Mathew A, Kumar S, Abraham OC, Trowbridge P, Mathai E. Differential diagnosis of scrub typhus meningitis from bacterial meningitis using clinical and laboratory features. Neurol India. 2013;61:17–20. DOIPubMedGoogle Scholar
- Pan S, Islam K, Datta A. Orientia tsutsugamushi: an emerging major cause of acute encephalitis syndrome in south Asian children. In: Abstracts of the British Paediatric Allergy Immunity and Infection Group; 2021. Abstract 989. Archives of Disease in Childhood, 2021 [cited 2022 Jul 1]. https://adc.bmj.com/content/archdischild/106/Suppl_1/A185.full.pdf
- Kaur P, Jain R, Kumar P, Randev S, Guglani V. Clinical spectrum and outcome of acute encephalitis syndrome in children with scrub typhus: a series of eight cases from India. Indian J Crit Care Med. 2020;24:885–7. DOIPubMedGoogle Scholar
- Ralph R, Prabhakar AT, Sathyendra S, Carey R, Jude J, Varghese GM. Scrub typhus-associated opsoclonus: clinical course and longitudinal outcomes in an Indian cohort. Ann Indian Acad Neurol. 2019;22:153–8.PubMedGoogle Scholar
- Saini L, Dhawan SR, Madaan P, Suthar R, Saini AG, Sahu JK, et al. Infection-associated opsoclonus: a retrospective case record analysis and review of literature. J Child Neurol. 2020;35:480–4. DOIPubMedGoogle Scholar
- Nandi M, Maity D. Opsoclonus myoclonus syndrome: a presenting feature of scrub typhus in a child. The Child and Newborn. 2018;22:8–9.
- Cho HJ, Choi JH, Sung SM, Jung DS, Choi KD. Bilateral optic neuritis associated with scrub typhus. Eur J Neurol. 2013;20:e129–30. DOIPubMedGoogle Scholar
- Jessani LG, Gopalakrishnan R, Kumaran M, Devaraj V, Vishwanathan L. Scrub typhus causing unilateral optic neuritis. Indian J Pediatr. 2016;83:1359–60. DOIPubMedGoogle Scholar
- Sood AK, Chauhan L, Gupta H. CNS manifestations in Orientia tsutsugamushi disease (scrub typhus) in North India. Indian J Pediatr. 2016;83:634–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 17, 2023
1These authors contributed equally to this article.
Table of Contents – Volume 29, Number 4—April 2023
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Tina Damodar, Department of Neurovirology, National Institute of Mental Health & Neurosciences, Bangalore 560029, India
Top