Volume 29, Number 6—June 2023
Research Letter
Microscopic Evidence of Malaria Infection in Visceral Tissue from Medici Family, Italy
Abstract
Microscopy of mummified visceral tissue from a Medici family member in Italy identified a potential blood vessel containing erythrocytes. Giemsa staining, atomic force microscopy, and immunohistochemistry confirmed Plasmodium falciparum inside those erythrocytes. Our results indicate an ancient Mediterranean presence of P. falciparum, which remains responsible for most malaria deaths in Africa.
The Medici family was a powerful family from Florence, Italy, that gained prominence under Cosimo de’ Medici in the early 15th century (1). Dynastic power granted Medici family members a burial at the San Lorenzo Basilica in central Florence (Appendix Figure 1, panel A). Burial was preceded by an embalming procedure in which inner organs (viscera) were removed and placed in large terracotta jars (Appendix Figure 1, panel B).
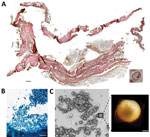
In 2011, selected jars of organs from Medici family members were opened centuries after burial to examine their contents, revealing that multiple tissue pieces were still present (Appendix). The Institute for Mummy Studies at Eurac Research (Bolzano, Italy) received samples from the organs; we performed microscopic and molecular analysis (Appendix) of a 2.5 cm × 1.5 cm tissue piece (ID 1297) from 1 jar (Appendix Figure 1, panel C). Using microscopy, we identified a potential blood vessel containing erythrocytes (Figure, panel A). Diameters (7.24, SD ±0.14 µm; n = 37) and discocyte shapes of cells within the blood vessel were characteristic of erythrocytes (2). We conducted further microscopic evaluation of single cells and found the potential presence of a parasite that might have resided within the erythrocytes during the lifetime of the deceased family member. Giemsa staining of tissue sections confirmed our first impression (Figure, panel B) and suggested the parasite was Plasmodium spp.; members of this genus are the causative agent of different types of human malaria (3). We used atomic force microscopy to identify the ring stage, an immature developmental stage of P. falciparum that is dominant in peripheral blood of infected patients and a diagnostic hallmark (Figure, panel C). We verified the presence of P. falciparum by using immunohistochemistry with polyclonal mouse antiserum against Plasmodium spp.–specific aldolase (Appendix Figure 1, panels D, E) and a monoclonal antibody against P. falciparum–specific histidine-rich protein HRPII (Appendix Figure 1, panels F, G). We confirmed results by using immunofluorescence analysis with antibody against P. falciparum endoplasmic reticulum resident protein Pf39 (Appendix Figure 1, panels H, I). All isotype controls were negative (Appendix Figure 1, panels E, G, I). Not all observed parasitized erythrocytes were labeled by the antiserum, likely because of tissue degradation over the centuries. We verified a progressed state of biomolecule degradation by additional DNA-based analysis.
We determined that parasitemia was 38% in the Medici tissue, which appeared high (Appendix Figure 1, panel J). However, instead of peripheral blood, we investigated tissues that might have had higher than expected parasitemia from sequestration of erythrocytes parasitized by mature asexual developmental stages (trophozoite and schizonts) of P. falciparum (4). Erythrocytes were visible in the tissue and were not washed away after embedding, further suggesting the presence of malaria parasites because they can trigger blood coagulation that might have kept the cells in place (5). High parasitemia within tissues is likely dependent on P. falciparum developmental stages (4). Erythrocytes infected with juvenile ring stages can be found in the peripheral blood of patients, whereas mature developmental stages are absent (6). Erythrocytes that contain more mature developmental stages can adhere to endothelial cells that line blood vessels within inner organs (6).
The most striking parasite-derived erythrocyte modification is the establishment of secretory organelles, known as Maurer’s clefts, that reside within the cytoplasm of terminally differentiated host erythrocytes infected with P. falciparum (7). Similar organelles also exist in the cytoplasm of erythrocytes infected by other pathogenic Plasmodium spp. (7). During P. falciparum infections, Maurer’s clefts are crucial for initiating host-parasite interactions; they are responsible for severe disease and patient death by enabling protein trafficking that causes cytoadherence within organs (4). By using Giemsa staining, we observed delicate stipplings within the cytoplasm of infected erythrocytes in the Medici tissue, indicative of Maurer’s clefts (Appendix Figure 1, panel J). We quantified the stipplings; numbers were comparable to what can be observed within infected erythrocytes of malaria patients and in vitro–infected erythrocyte cultures.
We performed glycan analysis by using mass spectrometry and molecular analyses (Appendix). We identified a unique glycan found in erythrocyte B antigen (Appendix Figure 2, panels A–D), further indicating the presence of erythrocytes in the tissue. However, parasite DNA was undetectable by PCR. Metagenomic sequencing showed only 0.06% of all reads were host DNA; 2 reads could be unambiguously assigned to P. falciparum (Appendix Figure 2, panel E).
Medici family members were known to hunt in marshlands around Florence and in Tuscany that served as breeding grounds for mosquito vectors capable of transmitting Plasmodium spp. parasites (8). In 2010, immunoassays were used to analyze bones of 4 Medici family members who might have died from malaria; P. falciparum was detected (9). Our observations agree with previous studies of ancient human remains, suggesting a Mediterranean presence of malaria from the era of ancient Egypt to modern times (10). Malaria remains a major health threat for persons in Africa, mostly affecting pregnant women and children. Malaria is a curable disease; however, persons in malaria-endemic areas still lack access to proper healthcare. Developing Plasmodium resistance to standard treatments further hampers positive therapeutic outcomes.
Dr. Maixner is coordinator at the EURAC Institute for Mummy Studies and head of the ancient DNA laboratory. His research interests focus on genomic and relationship analysis of ancient human remains and identification of ancient pathogens that caused disease.
Acknowledgments
We thank Donatella Lippi and Elsa Pacciani for their support during the initial phase of the study.
Funding was provided by the European Regional Development Fund 2014–2020_CALL-FESR 2017 Research and Innovation_Autonomous Province of Bolzano-South Tyrol_Project: FESR1078-MummyLabs.
References
- The Medici: citizens and masters. In: Black R, Law JE, editors. Villa I Tatti Series 32, the Harvard University Center for Italian Renaissance Studies. Cambridge (MA): Harvard University Press; 2015.
- Kilian N, Dittmer M, Cyrklaff M, Ouermi D, Bisseye C, Simpore J, et al. Haemoglobin S and C affect the motion of Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. Cell Microbiol. 2013;15:1111–26. DOIPubMedGoogle Scholar
- World Health Organization. World malaria report 2021 [cited 2023 Jan 1]. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021
- Cyrklaff M, Sanchez CP, Kilian N, Bisseye C, Simpore J, Frischknecht F, et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science. 2011;334:1283–6. DOIPubMedGoogle Scholar
- Francischetti IMB, Seydel KB, Monteiro RQ. Blood coagulation, inflammation, and malaria. Microcirculation. 2008;15:81–107. DOIPubMedGoogle Scholar
- Lee WC, Russell B, Rénia L. Sticking for a cause: the falciparum malaria parasites cytoadherence paradigm. Front Immunol. 2019;10:1444. DOIPubMedGoogle Scholar
- Mundwiler-Pachlatko E, Beck HP. Maurer’s clefts, the enigma of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2013;110:19987–94. DOIPubMedGoogle Scholar
- Menning CB. The noble hunt: the “Libro della caccia” of Angelo del Bufalo. Yale Univ Libr Gaz. 2001;76:27–35.PubMedGoogle Scholar
- Fornaciari G, Giuffra V, Ferroglio E, Gino S, Bianucci R. Plasmodium falciparum immunodetection in bone remains of members of the Renaissance Medici family (Florence, Italy, sixteenth century). Trans R Soc Trop Med Hyg. 2010;104:583–7. DOIPubMedGoogle Scholar
- Boualam MA, Pradines B, Drancourt M, Barbieri R. Malaria in Europe: a historical perspective. Front Med (Lausanne). 2021;8:
691095 . DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 29, Number 6—June 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Albert Zink, Institute for Mummy Studies, Eurac Research, Viale Druso 1, 39100 Bolzano, Italy
Top