Volume 29, Number 7—July 2023
Dispatch
Nonnegligible Seroprevalence and Predictors of Murine Typhus, Japan
Abstract
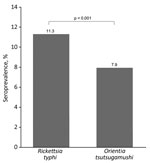
To elucidate the epidemiology of murine typhus, which is infrequently reported in Japan, we conducted a cross-sectional study involving 2,382 residents of rickettsiosis-endemic areas in Honshu Island during August–November 2020. Rickettsia typhi seroprevalence rate was higher than that of Orientia tsutsugamushi, indicating that murine typhus is a neglected disease.
Murine typhus (MT), a fleaborne rickettsiosis caused by the bacterium Rickettsia typhi, is a ubiquitous but clinically less recognizable disease than scrub typhus or spotted fever group rickettsioses (1). Limited testing because of the infection’s nonspecific symptoms and the need for expert laboratories for serodiagnosis makes MT an underrecognized infection. MT occurs worldwide and is endemic to warm urban or coastal regions where the climate is favorable for rats, which can serve as the reservoir of R. typhi. However, epidemiologic characteristics and risk factors often vary by region (1–3). Therefore, accumulating specific and local evidence from each region is required to elucidate the complete picture of MT epidemiology.
In Japan, MT with Xenopsylla cheopis fleas as the vector and Rattus rattus or R. norvegicus rats as the reservoir was endemic before the 1950s (4), but the disease has not been notifiable; only a few cases have been reported since then (5). As a consequence, the epidemiologic characteristics remain unknown, rendering MT an underrecognized and neglected infection. Clarifying the epidemiologic features of MT in Japan will help clinicians recognize the disease and provide early treatment. We estimate the seroprevalence of rickettsia, primarily of R. typhi, in rickettsia-endemic areas of Honshu Island (the largest island of Japan) and characterize the risk factors for MT.
We conducted a cross-sectional study in 3 sites in the southeastern part of Honshu (Boso Peninsula), endemic areas for scrub typhus and Japanese spotted fever (6). We included persons who underwent regular checkups during August–November 2020 (Appendix). Questionnaires were distributed during checkups, and the following data were collected: medical history of rickettsioses; environmental exposure to mountains, agriculture, and bushes; and residential addresses. The respondents were asked through questionnaires whether they resided in or had visited mountainous areas, had visited areas with small trees and weeds, or engaged in agricultural work. In addition, we measured the population density and area of each land use (coasts, forests, farmland, rivers or lakes, and wilderness) within a 500-meter radius of the participant’s address (Appendix). The study was approved by the Institutional Review Boards of Nagasaki University and Fukushima Medical University (approval nos. 200305230-2 and 2022-190). Written consent was obtained from all participants.
The primary outcomes were R. typhi seroprevalence and ratio of R. typhi to Orientia tsutsugamushi seroprevalence. O. tsutsugamushi was selected as the comparator outcome because scrub typhus is a notifiable disease and the rickettsiosis most endemic to Japan. Furthermore, we evaluated the seroprevalence of R. japonica, the pathogen of Japanese spotted fever, to determine the possibility of an apparently high seroprevalence of R. typhi because of cross-reactivity in the genus Rickettsia (7) (Appendix). We defined seropositivity as a ratio of >1:40 and defined O. tsutsugamushi seropositivity as a positive result for any of the O. tsutsugamushi serotypes. The sensitivity analyses estimated the seroprevalences at cutoff titers of 1:80 and 1:160.
Because the seropositivity rates of R. typhi and O. tsutsugamushi were regarded as paired binomial data, we tested the difference in their prevalence by using the McNemar test (8) and estimated it using conditional Poisson regression (Appendix). To explore the factors associated with R. typhi seropositivity, we fitted a logistic regression model by using the candidate risk factors. We assessed whether there were differences in the seroprevalence ratios across study sites and conducted the imputation of missing values (Appendix).
The median age of all participants was 67 years. R. typhi–seropositive participants exhibited a lower population density than R. typhi–seronegative participants, showing a similar trend to O. tsutsugamushi (Table 1; Appendix Table 1). The residential locations of R. typhi–seropositive participants were distributed throughout the Boso Peninsula, and a similar distribution was observed for O. tsutsugamushi–seropositive participants (Appendix Figures 1, 2). Although ≈60% of R. typhi–seropositive participants had titers of <160, 20 participants had titers of >1,280, and 4 had titers of >40,960 (Table 2). Most O. tsutsugamushi–seropositive participants had lower titers, although some exhibited notably high titers (Appendix Table 2).
R. typhi seroprevalence was 11.3% higher than that of O. tsutsugamushi (7.9%) (ratio of seropositivity 1.42; 95% CI 1.20–1.68) (Figure 1). Of the 2,382 residents, 204 were R. japonica seropositive, for a seroprevalence of 8.6%, lower than that of R. typhi. Furthermore, the antibody titer for R. typhi was higher than that for R. japonica in participants who were seropositive for both R. typhi and R. japonica (Appendix Table 3). Results of the sensitivity analyses did not show any changes to the predominance of the R. typhi seroprevalence over the O. tsutsugamushi seroprevalence (Appendix Table 4).
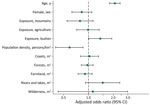
According to the multivariate analysis (Figure 2), the factors associated with R. typhi seropositivity were age (per 10-year increase; adjusted odds ratio [aOR] 2.09 [95% CI 1.80–2.42]), low population density (per 1,000 persons/km2 increase; aOR 0.59 [95% CI 0.40–0.86]), and history of bush exposure (aOR 1.39 [95% CI 1.01–1.92]) (Appendix).
We demonstrated robust findings of the predominance of R. typhi seroprevalence over O. tsutsugamushi seroprevalence in rickettsia-endemic areas in Japan. Previously, in those study areas, an epidemiologic study was conducted using now antiquated serologic methods, such as the Weil–Felix test (4), which provided unreliable MT and scrub typhus seroepidemiologic data (9). Contrary to the previous study’s findings, we were able to estimate rickettsiosis seroprevalence and confirm the predominance of MT more precisely using the standard diagnostic test.
This study illustrated that MT is a prevalent and possibly reemerging infection in Japan. Recently, in the United States (10), Greece (11), and Spain (12), the incidence of MT has increased, partly because of improved disease recognition (10) and a change in the transmission route (13). Thus, given the high seroprevalence of R. typhi in Japan, case accumulation is crucial to clarify the possibility of a unique transmission cycle.
The risk factors for R. typhi seropositivity in this study differed from those in previous studies. The increase in R. typhi seroprevalence with decrease in residential population density contradicts the findings of previous studies that showed urban environment as a risk factor (2,14). In addition, exposure to weeded areas was identified as a risk factor, but residential environments, including those near coasts, rivers, and lakes, which have been reported as risk factors (2,3), were not correlated. The differences in risk factors between this study and previous studies might reflect differences in factors related to contact with vectors and reservoirs at each study site.
The first limitation of our study is that seropositivity to R. typhi could indicate cross-reactivity to R. japonica. However, because R. typhi seropositivity was higher than R. japonica seropositivity and the cross-reactivity rate to R. typhi in confirmed Japanese spotted fever patients is ≈20% (15), most patients with R. typhi seropositivity have a true MT infection. Second, we did not consider cross-reactivity within the same group (spotted fever or typhus group) in the genus Rickettsia. However, other diseases caused by this genus have been reported infrequently, except for Japanese spotted fever and MT in domestic infection cases. Third, this study was conducted in persons undergoing routine checkups and might not represent seroprevalence in the general population.
In summary, R. typhi seroprevalence was higher than that of O. tsutsugamushi in rickettsia-endemic areas of Japan, indicating that MT is a neglected and underrecognized condition. This study highlights the need to include MT in the differential diagnosis when examining patients with nonspecific infectious symptoms who are residing in rickettsia-endemic areas. Clinicians should consider comprehensive examinations for rickettsial infections, including MT testing, especially in those with a history of residence in sparsely populated areas or exposure to bushes.
Dr. Aita is an internist specializing in general internal medicine and clinical epidemiology. He belongs to the Department of General Internal Medicine, Fukushima Medical University as a teaching/research associate, and conducts research on the epidemiology of diseases, diagnostic accuracy, and infections, such as rickettsioses.
Acknowledgments
We thank the staff of the municipal offices in Otaki and Katsuura and Kameda Medical Center for collecting questionnaires and blood samples. We also thank Dr. Fujita, who made great efforts to conduct this research but died before its publication.
This work was supported by JSPS KAKENHI (grant no. JP 19K23972).
References
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913–8. DOIPubMedGoogle Scholar
- Devamani CS, Schmidt WP, Ariyoshi K, Anitha A, Kalaimani S, Prakash JAJ. Risk factors for scrub typhus, murine typhus, and spotted fever seropositivity in urban areas, rural plains, and peri-forest hill villages in South India: a cross-sectional study. Am J Trop Med Hyg. 2020;103:238–48. DOIPubMedGoogle Scholar
- Tamiya T. Recent advances in studies of tsutsugamushi disease in Japan. Tokyo: Medical Culture, Inc.; 1962. p. 53–54.
- Sakaguchi S, Sato I, Muguruma H, Kawano H, Kusuhara Y, Yano S, et al. Reemerging murine typhus, Japan. Emerg Infect Dis. 2004;10:964–5. DOIPubMedGoogle Scholar
- Sando E, Suzuki M, Katoh S, Fujita H, Taira M, Yaegashi M, et al. Distinguishing Japanese spotted fever and scrub typhus. Emerg Infect Dis. 2018;24:1633–41. DOIPubMedGoogle Scholar
- Uchiyama T, Zhao L, Yan Y, Uchida T. Cross-reactivity of Rickettsia japonica and Rickettsia typhi demonstrated by immunofluorescence and Western immunoblotting. Microbiol Immunol. 1995;39:951–7. DOIPubMedGoogle Scholar
- Pembury Smith MQR, Ruxton GD. Effective use of the McNemar test. Behav Ecol Sociobiol. 2020;74:133. DOIGoogle Scholar
- Stewart AG, Stewart AGA. An update on the laboratory diagnosis of rickettsia spp. infection. Pathogens. 2021;10:1319. DOIPubMedGoogle Scholar
- Ruiz K, Valcin R, Keiser P, Blanton LS. Rise in murine typhus in Galveston County, Texas, USA, 2018. Emerg Infect Dis. 2020;26:1044–6. DOIPubMedGoogle Scholar
- Labropoulou S, Charvalos E, Chatzipanagiotou S, Ioannidis A, Sylignakis P, Τaka S, et al. Sunbathing, a possible risk factor of murine typhus infection in Greece. PLoS Negl Trop Dis. 2021;15:
e0009186 . DOIPubMedGoogle Scholar - Rodríguez-Alonso B, Almeida H, Alonso-Sardón M, Velasco-Tirado V, Robaina Bordón JM, Carranza Rodríguez C, et al. Murine typhus. How does it affect us in the 21st century? The epidemiology of inpatients in Spain (1997-2015). Int J Infect Dis. 2020;96:165–71. DOIPubMedGoogle Scholar
- Penicks A, Krueger L, Morgan T, Nguyen K, Campbell J, Fogarty C, et al. Jumping into the future: an analysis of 50 years of flea data from mammalian wildlife collected during three flea-borne rickettsioses surveys in Orange County, 1967–2017. Proc Pap Annu Conf Mosq Vector Control Assoc Calif. 2019;87:1.
- Yao Z, Tang J, Zhan FB. Detection of arbitrarily-shaped clusters using a neighbor-expanding approach: a case study on murine typhus in south Texas. Int J Health Geogr. 2011;10:23. DOIPubMedGoogle Scholar
- Aita T, Sando E, Katoh S, Hamaguchi S, Fujita H, Kurita N. Serological cross-reactivity between spotted fever and typhus groups of rickettsia infection in Japan. Int J Infect Dis. 2023;130:178–81. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 09, 2023
Table of Contents – Volume 29, Number 7—July 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eiichiro Sando, Department of General Internal Medicine and Clinical Infectious Diseases, Fukushima Medical University, 1 Hikarigaoka, Fukushima city, Fukushima, 960-1295, Japan
Top