Volume 8, Number 11—November 2002
THEME ISSUE
Tuberculosis Genotyping
Tuberculosis Genotyping Network, United States
Human Exposure following Mycobacterium tuberculosis Infection of Multiple Animal Species in a Metropolitan Zoo
Abstract
From 1997 to 2000, Mycobacterium tuberculosis was diagnosed in two Asian elephants (Elephas maximus), three Rocky Mountain goats (Oreamnos americanus), and one black rhinoceros (Diceros bicornis) in the Los Angeles Zoo. DNA fingerprint patterns suggested recent transmission. An investigation found no active cases of tuberculosis in humans; however, tuberculin skin-test conversions in humans were associated with training elephants and attending an elephant necropsy.
Outbreaks of Mycobacterium tuberculosis have been documented in environments such as hospitals, schools, factories, homeless shelters, and prisons (1–5). In more unconventional settings, such as circuses and exotic animal facilities, outbreaks pose unique tuberculosis (TB) control challenges because transmission may involve animals as well as humans (6,7). Zoos are a particular public health concern because of the close contact between TB-susceptible animals and humans, specifically animal handlers and visitors to the facility or exhibit. Infection and disease related to M. tuberculosis have been reported for a variety of species ranging from birds to primates (8–10). Although evidence for human-to-animal transmission of M. tuberculosis has been described (11), little documentation of zoonotic transmission to humans exists (7). We describe the first reported multispecies epizootic of genotypically identical strains of M. tuberculosis in a zoo and the results of an investigation of exposed zoo employees.
From 1997 to 2000, M. tuberculosis was identified in six animals at the Los Angeles Zoo. In March 1997, an Asian elephant (Elephas maximus) (elephant 1) died of salmonellosis. During the necropsy, pulmonary lesions were discovered, and a lymph node specimen showed M. tuberculosis. In April 1997, a positive trunk wash culture of M. tuberculosis was obtained from a second Asian elephant (elephant 2), which had resided in the same barn as elephant 1. In July 1998, a Rocky Mountain goat (Oreamnos americanus) (goat 1) suffered deterioration associated with worsening pneumonia; the pathologic examination was consistent with TB, and culture confirmed M. tuberculosis. Tuberculin skin tests of two cohabiting goats (goats 2 and 3) were positive, but their cultures were negative. In August 1998, a black rhinoceros (Diceros bicornis) had a positive M. tuberculosis culture. In February 2000, routine chest radiographs of goats 2 and 3 showed abnormalities consistent with TB. We isolated M. tuberculosis from both animals.
We examined medical and location histories of the affected animals as well as handling practices, health-care procedures, and other means of potential exposure to M. tuberculosis. An epidemiologic link was defined as documented exposure to an infectious human or animal with TB. We conducted an infection control assessment of the animal compounds and health-care facilities and measured air flow in the compounds by smoke testing (12).
Elephant isolates (e.g., trunk washes) were obtained according to United States Department of Agriculture guidelines (13). We used saline nasal washes to gather rhinoceros isolates and tracheal washes to gather isolates from goats, as well as specimens for pathologic examination. The Los Angeles County Public Health Laboratory performed restriction fragment length polymorphism (RFLP) analyses on the isolates. Southern blots of PvuII–restricted chromosomal DNA were run in 1% agarose gels, probed with a DNA fragment corresponding to IS6110, and detected by chemiluminescence (14).
Employee TB Screening
Medical records of zoo employees were reviewed for evidence of TB symptoms (i.e., persistent cough, hemoptysis, night sweats, difficulty in breathing, and weight loss), chest radiograph information, and tuberculin skin-test results. In addition, a list of current and former employees was confidentially matched against reported TB cases in the California state registry from 1985 to 2000 (15). During an annual occupational health screening in June 2000, employees participated in TB symptom reviews and received tuberculin skin tests; they also completed a questionnaire on medical history, job type, and history of contact with the infected animals.
Tuberculin Skin-Test Conversion Categories and Statistical Analyses
A positive tuberculin skin test was defined with a documented induration of >5 mm. We categorized employees with positive tuberculin skin tests as true, probable, or possible converters or as nonconverters. True converters were patients with a negative two-step test (within a 3-week period), followed by an increase in induration of >10 mm within 2 years. Probable converters had no two-step test but had either an induration increase of >10 mm within a 2-year period or two negative (<5 mm) results within 1 year followed by a positive result of >10 mm. Possible converters had an initial negative result followed by a positive test. Nonconverters were patients with positive tuberculin skin tests who did not fit these conversion categories (e.g., one positive tuberculin skin test without a previous test). The questionnaire responses of converters (true, probable, and possible combined) were compared to those of employees with negative tuberculin skin tests.
Relative risk (RR) ratios were calculated by chi-square or Fisher’s exact test by using Epi Info 6 (Centers for Disease Control and Prevention, Atlanta, GA). Statistical significance was considered to be p<0.05.
Both elephants with TB had resided at the same exotic animal facility in the United States before arriving at the Los Angeles Zoo in 1994. In 1997, M. tuberculosis was found in four other elephants at the exotic animal facility; however, the RFLP pattern differed from that of elephants 1 and 2 (unpub. data). The only documented epidemiologic links among the affected animals were between the two elephants and among the three goats. No common contact outside the animal compounds and no contact with an infectious human was found to account for TB transmission among multiple species.
Standard operating procedures at the zoo included guidelines for animal quarantine and the use of N95 respirators during medical procedures. The elephant compound was 27 m from the rhinoceros compound, and the goat compound was 90 m from both. Smoke tests of the animal compounds showed adequate air movement of 0.3–0.9 m/s and winds of 4.8–8.0 km/hr in ambient conditions.
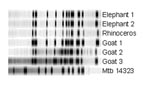
RFLP analysis showed that five of six animal isolates shared an identical 13-band IS6110 pattern (Figure). The isolate of goat 3 differed by one additional band.
No active TB cases in humans were found during employee TB screening, medical records review, or query of the state case registry. Of 1,088 employees, no matches were identified in the database of cases reported from 1985 to 2000.
Of 336 employees screened for TB during this investigation, 332 (99%) completed the questionnaire, and 307 (91%) had a tuberculin skin test. Of the 323 employees who responded to the job category question, most were veterinarians or animal keepers (112 [35%]) and administrative staff (73 [23%]); other job categories included maintenance workers (38 [12%]), custodians (29 [9%]), and groundskeepers (24 [7%]). Sixty-two percent of the animal handlers were Caucasian, and 61% of the groundskeepers were Hispanic. Sixty percent of all employees reported contact with animals.
Of the 307 employees who had tuberculin skin tests, 55 (18%) reported a positive result. Of these, none reported TB symptoms, and chest radiographs showed no abnormalities suggesting active TB. Persons with positive tuberculin skin tests were more likely than persons with negative tests to be men (RR 3.7, 95% confidence interval [CI] 2.0% to 6.8%), groundskeepers (RR 2.6, 95% CI 1.5% to 4.7%), construction workers (RR 2.5, 95% CI 1.3% to 4.8%), or attendees at the elephant necropsy (RR 2.9, 95% CI 1.5% to 5.5%). However, animal caretaking and animal contact were not associated with a positive tuberculin skin test. In this group of employees, we found no true converters, 10 (18%) probable converters, 5 (9%) possible converters, and 40 (73%) nonconverters.
Risk factors for tuberculin skin-test conversion are described in the table. Employees reporting attendance at elephant 1’s necropsy were more likely to have documented tuberculin skin-test conversions than those not present (RR 6.3, 95% CI 2.1% to 18.9%). Furthermore, employees who trained elephants were more likely to have tuberculin skin-test conversions than those who did not train elephants (RR 4.1, 95% CI 1.3% to 13.1%). Groundskeepers (n=24) had an increased risk of tuberculin skin-test conversion compared with other job categories (RR 7.1, 95% CI 2.6% to 19.1%). Four of five groundskeepers with tuberculin skin-test conversions were born in the United States; 11 of 14 employees with negative skin tests and none of the 5 groundskeepers with positive tuberculin skin tests (in the nonconverter group) were born in the United States. A lower likelihood of tuberculin skin-test conversion was associated with visiting the animal nursery (RR 0.2, 95% CI 0.0% to 0.7%) and the health center (RR 0.3, 95% CI 0.1% to 0.9%).
Although 55 zoo employees showed evidence of M. tuberculosis infection, no person with active TB disease was identified. Given the public’s distance from the animals and the absence of active TB among employees with closer contact with these animals, M. tuberculosis was likely not transmitted from humans to animals at this zoo.
The finding that groundskeepers and not animal handlers were associated with a higher risk of tuberculin skin-test conversion was unexpected. Because groundskeepers as a group were more likely to be born outside of the United States than animal keepers, we hypothesized that tuberculin skin-test conversion may have resulted from infections acquired outside of the zoo. However, within this group, only one of five groundskeepers with a tuberculin skin-test conversion was born outside of the country. This finding suggests that a recent exposure may have been responsible for tuberculin skin-test conversion in this occupational category, although small numbers limit the inference.
Genotyping evidence strongly suggested transmission from one species to another, although corroborating epidemiologic evidence of transmission was not discovered. One explanation for transmission is that the elephants may have been exposed to TB at the animal facility in which they resided before their arrival at the zoo. The distances to other animal compounds at the zoo make airborne spread unlikely. In addition, we found no employees with active TB. Since interspecies transmission routes were not found, we suggest that continued vigilance for sources of ongoing transmission is warranted.
Finally, we did discover a significant association between tuberculin skin-test conversion and attending the elephant necropsy and training elephants in the compound. This report emphasizes the importance of adhering to strict infection control measures during large animal necropsies and medical procedures, even when TB is not suspected, because of potentially large bacillary loads.
Mr. Oh is an epidemiologist in the TB Control Branch, Division of Communicable Disease Control at the California Department of Health Services. His main interests are TB surveillance, outbreak investigations, and international health.
Acknowledgments
We thank Charles Sedgwick and the zoo administrative office, staff veterinarians, animal health and animal care staff, occupational health nurses, and zoo employees for their participation in the questionnaire. We thank the TB Control Program staff and David Sasai for conducting the ventilation assessment.
This investigation was supported by the Centers for Disease Control and Prevention cooperative agreement funds for the National Tuberculosis Genotyping and Surveillance Network.
References
- Edlin BR, Tokars JI, Grieco MH, Crawford JT, Williams J, Sordillo EM, An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–21.PubMedGoogle Scholar
- Hoge CW, Fisher L, Donnell HD, Dodson DR, Tomlinson GV, Breiman RF, Risk factors for transmission of Mycobacterium tuberculosis in a primary school outbreak: lack of racial difference in susceptibility to infection. Am J Epidemiol. 1994;139:520–30.PubMedGoogle Scholar
- Mosher CB, Derebery VJ, Young BJ, Adams RA. Unusually aggressive transmission of tuberculosis in a factory. J Occup Med. 1987;29:29–31.PubMedGoogle Scholar
- Curtis AB, Ridzon R, Novick LF, Driscoll J, Blair D, Oxtoby M, Analysis of Mycobacterium tuberculosis transmission patterns in a homeless shelter outbreak. Int J Tuberc Lung Dis. 2000;4:308–13.PubMedGoogle Scholar
- Valway SE, Richards SB, Kovacovich J, Greifinger RB, Crawford JT, Dooley SW. Outbreak of multi-drug-resistant tuberculosis in a New York State prison, 1991. Am J Epidemiol. 1994;140:113–22.PubMedGoogle Scholar
- Darney PD, Greene JE. Tuberculosis outbreak in a circus: report of a cooperative investigation. Am J Public Health. 1973;63:43–5.PubMedGoogle Scholar
- Michalak K, Austin C, Diesel S, Bacon MJ, Zimmerman P, Maslow JN. Mycobacterium tuberculosis infection as a zoonotic disease: transmission between humans and elephants. Emerg Infect Dis. 1998;4:283–7.PubMedGoogle Scholar
- Ackerman LJ, Benbrook SC, Walton BC. Mycobacterium tuberculosis infection in a parrot (Amazona farinosa). Am Rev Respir Dis. 1974;109:388–90.PubMedGoogle Scholar
- Shin NS, Kwon SW, Han DH, Bai GH, Yoon J, Cheon DS, Mycobacterium tuberculosis infection in an orangutan (Pongo pygmaeus). J Vet Med Sci. 1995;57:951–3.PubMedGoogle Scholar
- Montali RJ, Mikota SK, Cheng LI. Mycobacterium tuberculosis in zoo and wildlife species. Rev Sci Tech. 2001;20:291–303.PubMedGoogle Scholar
- Michel AL, Huchzermeyer HF. The zoonotic importance of Mycobacterium tuberculosis: transmission from human to monkey. J S Afr Vet Assoc. 1998;69:64–5.PubMedGoogle Scholar
- Schwartzman K, Loo V, Pasztor J, Menzies D. Tuberculosis infection among health care workers in Montreal. Am J Respir Crit Care Med. 1996;154:1006–12.PubMedGoogle Scholar
- Department of Agriculture (US). Animal and Plant Health Inspection Service, Guidelines for the control of tuberculosis in elephants. Washington: The Department; 1997.
- van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–9.PubMedGoogle Scholar
- Yokoe DS, Subramanyan GS, Nardell E, Sharnprapai S, McCray E, Platt R. Supplementing tuberculosis surveillance with automated data from health maintenance organizations. Emerg Infect Dis. 1999;5:779–87.PubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 8, Number 11—November 2002
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jennifer Flood, California Department of Health Services, Tuberculosis Control Branch, 2151 Berkeley Way, Room 608, Berkeley, CA 94704, USA; fax: 510-849-5269;
Top