Volume 8, Number 7—July 2002
Research
Ehrlichia ewingii Infection in White-Tailed Deer (Odocoileus virginianus)
Abstract
Two closely related zoonotic ehrlichiae, Ehrlichia chaffeensis and E. ewingii, are transmitted by Amblyomma americanum, the lone star tick. Because white-tailed deer (Odocoileus virginianus) are critical hosts for all mobile stages of A. americanum and are important vertebrate reservoirs of E. chaffeensis, we investigated whether deer may be infected with E. ewingii, a cause of granulocytotropic ehrlichiosis in humans and dogs. To test for E. ewingii infection, we used polymerase chain reaction and inoculation of fawns with whole blood from wild deer. Of 110 deer tested from 20 locations in 8 U.S. states, 6 (5.5%) were positive for E. ewingii. In addition, natural E. ewingii infection was confirmed through infection of captive fawns. These findings expand the geographic distribution of E. ewingii, along with risk for human infection, to include areas of Kentucky, Georgia, and South Carolina. These data suggest that white-tailed deer may be an important reservoir for E. ewingii.
Ehrlichia ewingii, one of the causative agents of canine granulocytic ehrlichiosis, has been reported in dogs in several U.S. states, including Oklahoma, North Carolina, and Virginia (1–4). Human infections with E. ewingii have been reported from Missouri, Oklahoma, and Tennessee (5,6); the clinical disease, similar to that caused by other Ehrlichia spp., is characterized by fever, headache, and thrombocytopenia, with or without leukopenia (5–7). Experimentally, the lone star tick (Amblyomma americanum) has been shown to be a competent vector (8); however, natural infection of two other tick species, Rhipicephalus sanguineus and Dermacentor variabilis, has been reported in Oklahoma (2).
The white-tailed deer (Odocoileus virginianus) is an important host for all three mobile stages of A. americanum, and deer and lone star ticks serve as the major reservoir and vector, respectively, for E. chaffeensis (9–11). Because E. ewingii is closely related to E. chaffeensis and shares the same vector, our goal was to determine if white-tailed deer are naturally infected with E. ewingii. In some human and canine infections with E. ewingii, cross-reactions with E. chaffeensis antigens have been reported (5,6); however, not all infections with E. ewingii result in positive serologic tests to E. chaffeensis antigen (2,6). Because E. ewingii has not been isolated in culture and because serologic test reagents are not readily available, we used several techniques to detect infections, including 1) testing serum samples for antibodies reactive with E. chaffeensis antigen, 2) testing leukocytes or whole blood by polymerase chain reaction (PCR) with primers specific for E. ewingii and E. chaffeensis, and 3) injecting captive white-tailed fawns with whole blood from deer collected in an A. americanum–endemic area.
From September 1996 to July 2001, whole blood samples and serum from 110 deer from 20 sites (Table 1) in the southeastern United States were collected in vacutainer EDTA tubes (whole blood) and serum tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). For PCR, two blood preparation protocols were followed. During the 1996–1997 collection period, leukocytes were separated from whole blood as described (9); during the 2000–2001 period, whole blood was extracted for PCR assays. Both leukocytes and whole blood samples were frozen at –20°C until PCR testing was done. Serum samples were held in vials at –20°C until serologic testing.
Because A. americanum is the only experimentally proven vector for E. ewingii, locations with deer infested with A. americanum were selected for this study. Serum from each deer was tested for antibodies reactive to E. chaffeensis by the indirect immunofluorescent antibody (IFA) test as described (10), with the following modifications. Briefly, sera were screened at a dilution of 1:128 by using E. chaffeensis antigen slides obtained from Focus Technologies (formerly MRL Diagnostics, Cypress, CA). A 1:50 dilution of fluorescein isothiocyanate-labeled rabbit anti-deer immunoglobulin G (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) was used as conjugate.
DNA from 200 µL whole blood or 20 µL leukocytes was extracted by using the GFX Genomic Blood DNA Purification Kit (Amersham Biosciences, Piscataway, NJ) and InstaGene Purification Matrix (Bio-Rad Laboratories, Hercules, CA), respectively, following the manufacturer’s protocol. Primary outside amplification consisted of 5 µL DNA from whole blood or 10 µL from leukocytes in a 25-µL reaction containing 10 mM Tris-Cl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate (dNTP), and 2.5 units Taq DNA Polymerase (Promega Corp., Madison, WI), and 0.8 µM of primers ECC and ECB (11). For the nested PCR, 1 µL of primary product was used as template in a 25-µL reaction containing the same PCR components, except for the addition of E. ewingii specific primers, EE72-ewingii (5´-CAATTCCTAAATAGTCTCTGACTATT-3´) and HE3 (4), or E. chaffeensis-specific primers, HE1 and HE3 (11). Amplified products were separated in 2% agarose gels, stained with ethidium bromide, and visualized with UV light. Representative secondary PCR products for E. ewingii were purified with a Microcon spin filter (Amicon Inc., Beverley, MA), sequenced with an ABI 3100 automated sequencer (Applied Biosystems, Perkin Elmer Corp, Foster City, CA), and then compared with published E. ewingii sequences (GenBank accession nos. M73227 [3] and U96436 [1]).
Two 4-month-old, laboratory-reared white-tailed fawns (76 and 81) were housed in a tick-free facility. Before inoculation both fawns were negative for antibodies reactive to E. chaffeensis and PCR-negative for both E. chaffeensis and E. ewingii. Whole blood for injection was obtained from five wild source deer (WTD 1–5) collected at Piedmont National Wildlife Refuge (NWR) in Jones County, Georgia, on July 24, 2001. A whole blood sample from each wild deer was also cultured in DH82 canine macrophage cells as described (12).
Fawns were anesthetized by intramuscular injection of tiletamine HCL and zolazepam HCL (4.4 mg/kg body weight; Fort Dodge Animal Health, Fort Dodge, IA) and xylaxine (2.2 mg/kg; Butler, Columbus, OH) and were reversed with intravenous injection of yohimbine (0.125 mg/kg; Lloyd Laboratories, Inc., Shenandoah, IA). Equal volumes of whole blood in EDTA from WTD1 and WTD2 were pooled, and a total of 8 mL was injected into fawn 76 in 2-mL aliquots by each of four routes (intravenous, intradermal, subcutaneous, and intraperitoneal). Fawn 81 was injected in the same way with a total of 8 mL of pooled blood from WTD3–5. Blood samples were collected from both fawns on 5, 9, 15, 20, 47, 68, and 110 days postinjection (DPI) for PCR, serologic tests, and blood smears. Blood was tested by PCR for E. ewingii and E. chaffeensis as described above and for the human granulocytic ehrlichiosis (HGE) agent (Anaplasma phagocytophila) by using primers GE9f and GA1UR, as described (13).
Ninety-seven (88.1%) of the 110 wild deer had antibodies reactive (≥1:128 titer) to E. chaffeensis by IFA testing. All locations examined contained seropositive deer (range 57%–100%). A 407-bp product characteristic of E. ewingii was generated in six (5.5%) deer by nested PCR, and six (5.5%) deer were also positive for E. chaffeensis (Table 1). Positive PCR results for E. chaffeensis and E. ewingii were obtained with both blood preparation processes. Only one deer (0.9%) was positive for both E. ewingii and E. chaffeensis by PCR.
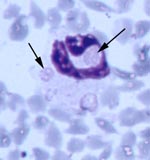
All five source deer (WTD1–5) were positive for antibodies to E. chaffeensis, but negative by PCR for E. ewingii and E. chaffeensis (Table 2). However, blood from deer WTD5 was culture positive for E. chaffeensis. Fawn 81 was at first positive for antibodies reactive to E. chaffeensis at 15 DPI, tested negative at 20 DPI, and was positive at 47, 68, and 110 DPI. Fawn 76 was seronegative on all days tested. Both fawns were PCR positive for E. ewingii at 47 DPI, and fawn 81 remained PCR positive at 68 DPI (Table 2). Whole blood samples from fawn 81 were PCR positive for E. chaffeensis at 15, 20, 47, 68, and 110 DPI. On thin blood smears taken at 47 DPI, morulae characteristic of E. ewingii were observed in approximately 2%-3% and <1% of neutrophils of fawns 81 and 76, respectively (Figure). Both deer remained PCR negative for the HGE agent.
Sequences of three E. ewingii products (Dare County, North Carolina; Fawn 76; and Fawn 81) were identical to published gene sequences M73227 and U96436. The E. ewingii product from Benton County, Arkansas, differed from the others at base 225, which corresponds to GenBank accession number AY093439. The E. ewingii sequences were deposited in the GenBank database under accession numbers AY093439–AY093441 and AY497628.
Our data provide the first evidence that white-tailed deer are naturally infected with E. ewingii; this information extends the geographic distribution of E. ewingii to include areas of Kentucky, Georgia, and South Carolina. Before this report, the only reported vertebrate hosts for E. ewingii were humans and dogs. By combining data from PCR and injection studies, we showed that at least 8 (7.3%) of 110 deer were infected with E. ewingii, which is similar to prevalence rates previously reported for dogs. Infection with E. ewingii has been reported in 6.2%-15.8% of dogs from southeastern Virginia, Oklahoma, and southeastern North Carolina (2,4,14). Because of the limited sensitivity of PCR for detection of this organism, this percentage may represent a substantial underestimation of the actual prevalence of E. ewingii infection in white-tailed deer. Our data suggest that the distribution of E. ewingii and hence the risk for human and canine infection may be more widespread than previously reported and may correspond with the distribution of A. americanum.
Although whole blood samples from all five deer (WTD1–5) collected at Piedmont NWR in Georgia were negative by PCR, Ehrlichia spp. infections developed in both inoculated fawns. Therefore, at least two of the Piedmont NWR deer were infected with E. ewingii, since E. ewingii infection was identified in both fawns. In addition, at least one Piedmont NWR deer was positive for E. chaffeensis, as fawn 81 became infected and WTD5 was culture positive. Because a much smaller volume of blood was used for PCR (20–200 µL) than for culture (5 mL) or injection of fawns (8 mL), low numbers of organisms may have been more readily detected by the other two methods. Consistent with results of previous studies (12,15), our data indicate that use of PCR alone as a screening tool may fail to detect acute infections of white-tailed deer with Ehrlichia spp.
Although fawn 76 was clearly infected with E. ewingii on the basis of PCR and detection of morulae, its results were never positive by serology. Serologic cross-reactions between E. ewingii and E. chaffeensis have been reported (5,6); however, not all E. ewingii-infected dogs or humans develop antibodies to E. chaffeensis antigens (2,6). Compared with previous experimental infections of white-tailed deer with E. chaffeensis (11,15), an extended period of time was required before E. ewingii was detected. Low numbers of E. ewingii in the original inoculum may explain the longer time required for PCR detection of E. ewingii in fawns 76 and 81. Because this experimental infection was a small pilot study, limited insight is provided into the course of E. ewingii infection in white-tailed deer. However, the detection of E. ewingii in fawn 81 over a 3-week period indicates that E. ewingii was capable of replicating in white-tailed deer.
White-tailed deer have been demonstrated as important reservoirs for E. chaffeensis (11,12,15). In this study, using PCR, culture, and inoculation of fawns, at least 7 (6.4%) of 110 deer were positive for E. chaffeensis. In previous studies in A. americanum–endemic areas, as many as 40%–100% of white-tailed deer have been shown to have antibodies reactive with E. chaffeensis, and up to 20% of deer are PCR positive (10,12). Five of the seven populations of white-tailed deer positive for E. chaffeensis were also positive for E. ewingii. This finding is not surprising, as these pathogens share the same vector. Although evidence of the HGE agent has been detected in white-tailed deer by both serologic testing and PCR (13,16), the relative importance of deer as reservoirs for the HGE agent has not been fully evaluated. Although our study demonstrates that white-tailed deer can harbor a third human ehrlichial pathogen, the importance of deer as a reservoir is not known.
Data from this study raise several important issues: 1) because of epidemiologic similarities between E. chaffeensis and E. ewingii, deer could be an important reservoir for E. ewingii; 2) because of potential serologic cross-reactivity, E. chaffeensis seroreactors in the current and prior surveys of white-tailed deer (10,17) could actually represent E. chaffeensis, E. ewingii, or mixed infections; and 3) because at least four Ehrlichia species infect white-tailed deer (E. chaffeensis, E. ewingii, A. phagocytophila, and an undescribed Ehrlichia sp.) (9,12,13,16), an array of diagnostic assays should be used for detecting Ehrlichia spp. infections. Therefore, further studies are needed to examine the reservoir potential of white-tailed deer for E. ewingii infection.
Mr. Yabsley is a doctoral student in the College of Veterinary Medicine at the University of Georgia. His area of research is the epidemiology of zoonotic parasites, with a particular focus on tick-borne pathogens.
Acknowledgments
The authors thank John Sumner for providing an Ehrlichia ewingii-positive DNA sample, M. Page Luttrell and Victor Moore for laboratory assistance, and the staff at Southeastern Cooperative Wildlife Disease Study for field and technical assistance.
This work was supported primarily by the National Institutes of Allergy and Infectious Diseases (5 R01 AI044235-02). Further support was provided by the Federal Aid to Wildlife Restoration Act (50 Stat. 917) and through sponsorship from fish and wildlife agencies in Alabama, Arkansas, Florida, Georgia, Kansas, Kentucky, Louisiana, Maryland, Mississippi, Missouri, North Carolina, Oklahoma, Puerto Rico, South Carolina, Tennessee, Virginia, and West Virginia.
References
- Goldman EE, Breitschwerdt EB, Grindem CB, Hegarty BC, Walls JJ, Dumler JS. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J Vet Intern Med. 1998;12:61–70.PubMedGoogle Scholar
- Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–39. DOIPubMedGoogle Scholar
- Anderson BE, Greene CE, Jones DC, Dawson JE. Ehrlichia ewingii sp. nov., the etiologic agent of canine granulocytic ehrlichiosis. Int J Syst Bacteriol. 1992;42:299–302.PubMedGoogle Scholar
- Dawson JE, Biggie KL, Warner CK, Cookson K, Jenkins S, Levine JF, Polymerase chain reaction evidence of Ehrlichia chaffeensis, an etiologic agent of human ehrlichiosis, in dogs from southeast Virginia. Am J Vet Res. 1996;57:1175–9.PubMedGoogle Scholar
- Buller RS, Arens M, Hmiel SP, Paddock CD, Sumner JW, Rikhisa Y, Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N Engl J Med. 1999;341:148–55. DOIPubMedGoogle Scholar
- Paddock CD, Folk SM, Shore GM, Machado LJ, Huycke MM, Slater LN, Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin Infect Dis. 2001;33:1586–94. DOIPubMedGoogle Scholar
- McQuiston JH, Paddock CD, Holman RC, Childs JE. Human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–42.PubMedGoogle Scholar
- Anziani OS, Ewing SA, Barker RW. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res. 1990;51:929–31.PubMedGoogle Scholar
- Little SE, Dawson JE, Lockhart JM, Stallknecht DE, Warner CK, Davidson WR. Development and use of specific polymerase reaction for the detection of an organism resembling Ehrlichia sp. in white-tailed deer. J Wildl Dis. 1997;33:246–53.PubMedGoogle Scholar
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE. Site-specific geographic association between Amblyomma americanum (Acari: Ixodidae) infestations and Ehrlichia chaffeensis-reactive (Rickettsiales: Ehrlicheae) antibodies in white-tailed deer. J Med Entomol. 1996;33:153–8.PubMedGoogle Scholar
- Dawson JE, Stallknecht D, Howerth EW, Warner C, Biggie KL, Davidson WR, Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol. 1994;32:2725–8.PubMedGoogle Scholar
- Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, Howerth EW. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35:1681–6.PubMedGoogle Scholar
- Little SE, Stallknecht DE, Lockhart JM, Dawson JE, Davidson WR. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. J Parasitol. 1998;84:897–901. DOIPubMedGoogle Scholar
- Kordick SK, Breitschwerdt EB, Hegarty BC, Southwick KL, Colitz CM, Hancock SI, Coinfection with multiple tick-borne pathogens in Walker Hound kennel in North Carolina. J Clin Microbiol. 1999;37:2631–8.PubMedGoogle Scholar
- Davidson WR, Lockhart JM, Stallknecht DE, Howerth EW, Dawson JE, Rechav Y. Persistent Ehrlichia chaffeensis infection in white-tailed deer. J Wildl Dis. 2001;37:538–46.PubMedGoogle Scholar
- Magnarelli LA, Ijdo JW, Stafford KC III, Fikrig E. Infections of granulocytic ehrlichiae and Borrelia burgdorferi in white-tailed deer in Connecticut. J Wildl Dis. 1999;35:266–74.PubMedGoogle Scholar
- Dawson JE, Childs JE, Biggie KL, Moore C, Stallknecht D, Shaddock J, White-tailed deer as a potential reservoir of Ehrlichia spp. J Wildl Dis. 1994;30:162–8.PubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 8, Number 7—July 2002
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Michael J. Yabsley, Wildlife Health Building, Southeastern Cooperative Wildlife Disease Study, College of Veterinary Medicine, University of Georgia, Athens, GA 30602, USA; fax: 706-542-5865;
Top