Volume 9, Number 9—September 2003
Dispatch
Co-trimoxazole–Sensitive, Methicillin-Resistant Staphylococcus aureus, Israel, 1988–1997
Abstract
Among bloodstream methicillin-resistant Staphylococcus aureus (MRSA) isolates from adult patients in a single hospital, susceptibility to co-trimoxazole increased progressively from 31% in 1988 to 92% in 1997 (p<0.0001). If also observed in other institutions, these findings should encourage the performance of a clinical trial of sufficient size to compare co-trimoxazole to vancomycin in treating MRSA infections.
Methicillin-resistant Staphylococcus aureus (MRSA) is a growing medical concern. During the last 2 decades, the rates of infections caused by MRSA increased among hospitalized patients in most developed countries (1). The aim of this study was to examine trends in antibiotic resistance of hospital-acquired bloodstream MRSA isolates from 1988 to 1997 in our institution.
Included in the analysis were all patients >18 years of age who had hospital-acquired bacteremia caused by S. aureus. The study took place at Rabin Medical Center, Beilinson Campus, Petach-Tikva, Israel, a 900-bed university hospital. Our center serves an urban population of approximately 1 million persons as both a first-line and tertiary facility. A prospective surveillance of all bacteremic episodes occurring at our medical center is performed continuously and, since 1988, has been incorporated into a computerized database for bacteremia. Episodes of bacteremia are detected by daily surveillance of the microbiology laboratory records, with an annual range of 700 to 900 episodes.
Antibiotic susceptibility was tested by using the disk diffusion technique on Mueller-Hinton agar, according to the procedures established by the National Committee for Clinical Laboratory Standards (NCCLS) (2). Plates were incubated at 30ºC for 18 h and 40 h for methicillin (5 μg/disk) and at 37°C for 18 h for other antibiotics. Bacteremia was considered to be hospital-acquired if it appeared 48 h after admission.
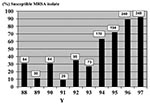
During the study period, a total of 944 episodes of S. aureus bacteremia were documented. We found 598 (63%) hospital-acquired episodes, with an annual number of episodes ranging from 35 to 121. Among the hospital-acquired episodes, 270 (45%) were due to MRSA strains. During the recent decade, rates of resistance to methicillin were high but stable among the hospital-acquired isolates, ranging from 25% to 57%. Rates of susceptibility to co-trimoxazole among patients with hospital-acquired MRSA increased significantly from 31% in 1988 to 92% in 1997 (p=0.0001) (Figure).
The hospital-acquired MRSA isolates were persistently highly resistant to chloramphenicol (69% in 1988 and 100% in 1997; p=NS), gentamicin (89% in 1988 to 94% in 1997; p=NS), and ciprofloxacin (87% in 1988 to 96% in 1997; p=NS). The resistance to clindamycin (62% in 1988 to 92% in 1997; p=0.04), fusidic acid (6% in 1988 to 14% in 1997; p=0.03), and rifampicin (21% in 1988 to 76% in 1997; p=0.02) increased significantly. All isolates were sensitive to vancomycin.
Our study shows that 92% of nosocomial MRSA strains were sensitive to co-trimoxazole in 1997 as compared with 31% in 1988. Several factors may have influenced the emergence of co-trimoxazole–sensitive MRSA, including the reduced usage of this drug in our institution. According to the pharmacy records, usage of co-trimoxazole in our institution decreased progressively from 28 daily doses per 1,000 hospital days in 1990 to 17 daily doses per 1,000 hospital days in 1997 (3). A recent multicenter report from several Belgian hospitals showed an increase in co-trimoxazole susceptibility among MRSA isolates (4). These findings are in contrast with trends of increasing resistance of S. aureus to a variety of anti-staphylococcal drugs other than co-trimoxazole, since the beginning of the antibiotic era. These trends had culminated recently with the appearance of glycopeptide resistance in hospitals and methicillin resistance in the community (5). Whether our findings reflect an increase of co-trimoxazole–sensitive MRSA clone/s in our institution needs further exploration. In settings where co-trimoxazole is extensively used, a substantial increase of MRSA resistance to co-trimoxazole has been observed. For example, Martin et al. described a serial cross-sectional study of resistance to co-trimoxazole among all clinical isolates of S. aureus and other Enterobacteriaceae during a 16-year period at San Francisco General Hospital (6). In this study, resistance to co-trimoxazole increased from 0% to 48% in S. aureus isolates obtained from HIV-infected patients. The authors explained this increase of resistance to co-trimoxazole by the extensive use of this drug as prophylaxis against Pneumocystis carinii pneumonia.
Eventually, our data may favor the use of co-trimoxazole as a potentially cost-effective antimicrobial drug for treating MRSA infections. Co-trimoxazole has been shown to be effective against MRSA both in vitro and in vivo in mice (7), as well as in clinical reports on meningitis, septicemia, and endocarditis (8,9). A controlled comparative trial of intravenous co-trimoxazole versus intravenous vancomycin in 101 cases of severe S. aureus infections in intravenous drug users was conducted by Markowitz et al. (10) in 1992. The authors reported 100% cure rates for either drug in MRSA infections, including bacteremia. More recently, Stein et al. showed varying degrees of success in treating with co-trimoxazole orthopedic implant infections caused by S. aureus (11). Unfortunately, this study did not distinguish MRSA from methicillin-sensitive S. aureus strains.
Recent in vitro data have shown good activity of co-trimoxazole against clinical isolates of vancomycin-intermediate S. aureus (12,13) and vancomycin-resistant S. aureus (14). In some of these cases, co-trimoxazole in combination with surgical debridement and other anti-staphylococcal drugs has been used successfully (12,14). In clinical practice, cyclical usage of co-trimoxazole and vancomycin and possible other newer anti-MRSA drugs such as oxazolidinones and streptogramins may prove of value in slowing down rates of development of antibiotic resistance in MRSA. The in vitro–presented results, if confirmed in other institutions, in conjunction with anecdotal clinical data, should encourage the performance of a clinical trial of sufficient size to compare co-trimoxazole to vancomycin in treating MRSA infections.
Dr. Bishara is a specialist in internal medicine and infectious diseases, and he serves as a senior physician and infectious disease consultant at the Rabin Medical Center, Israel. His major research interests are infective endocarditis and cardiovascular and nosocomial infections.
References
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, ; The SENTRY Participants Group. Clin Infect Dis. 2001;32:S114–32. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobials disk susceptibility tests. Approved standard M2-A4. Villanova (PA): The Committee; 1990.
- ATC index with DDDs. WHO Collaborating Centre for Drug Statistics Methodology. Oslo; 2001.
- Denis O, Deplano A, Nonhoff C, de Ryck R, Rottieres S, Hendricks E, Molecular epidemiology and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus in Belgian hospitals: 2001. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Abstract. San Diego: American Society for Microbiology; 2002. p. 305.
- Hiramatsu K, Okuma K, Ma XX, Yamamoto M, Hori S, Kapi M. New trends in Staphylococcus aureus infections: glycopeptide resistance in hospital and methicillin resistance in the community. Curr Opin Infect Dis. 2002;4:407–13.PubMedGoogle Scholar
- Martin JN, Rose DA, Hadley WK, Perdreau-Remington F, Lam PK, Gerberding JL. Emergence of trimethoprim-sulfamethoxazole resistance in the AIDS era. J Infect Dis. 1999;180:1809–18. DOIPubMedGoogle Scholar
- Elwell LP, Wilson HR, Knick VB, Keith BR. In vitro and in vivo efficacy of the combination of trimethoprim-sulfamethoxazole against clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1986;29:1092–4.PubMedGoogle Scholar
- Tamer MA, Bray JD. Trimethoprim- sulfamethoxazole treatment of multi-antibiotic-resistant staphylococcal endocarditis and meningitis. Clin Pediatr. 1982;21:125–6. DOIGoogle Scholar
- Sabel KG, Brandberg A. Treatment of meningitis and septicemia in infancy with a sulphamethoxazole trimethoprim combination. Acta Paediatr Scand. 1975;64:25–32. DOIPubMedGoogle Scholar
- Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulphamethoxazole compared with vancomycin for treatment of Staphylococcus aureus infection. Ann Intern Med. 1992;117:390–8.PubMedGoogle Scholar
- Stein A, Bataille JF, Drancourt M, Curvale G, Argenson JN, Groulier P, Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimethoprim-sulfamethoxazole). Antimicrob Agents Chemother. 1998;42:3086–91.PubMedGoogle Scholar
- Tsakris A, Papadimitriou E, Douboyas J, Stylianopoulou F, Manolis E. Emergence of vancomycin-intermediate Staphylococcus aureus and S. sciuri, Greece. Emerg Infect Dis. 2002;8:536–7.PubMedGoogle Scholar
- Close SJ, McBurney CR, Garvin CG, Chen DC, Martin SJ. Trimethoprim-sulfamethoxazole activity and pharmacodynamics against glycopeptide-intermediate Staphlococcus aureus. Pharmacotherapy. 2002;22:983–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:565–7.PubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 9, Number 9—September 2003
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
J. Bishara, Department of Internal Medicine C, Rabin Medical Center, Beilinson Campus, Petach Tivkah, Israel; fax: 972-3-922-1605
Top