Volume 12, Number 10—October 2006
Dispatch
Helminth-related Eosinophilia in African Immigrants, Gran Canaria
Abstract
Of 788 recent African adult immigrants to Las Palmas de Gran Canaria, 213 (27.0%) had eosinophilia. The most frequent causes were filariasis (29.4%), schistosomiasis (17.2%), and hookworm infection (16.8%). Stool microscopy and filarial and schistosomal serologic tests gave the highest diagnostic yield. Country of origin and eosinophil count were associated with specific diagnoses.
We prospectively evaluated the prevalence and causes of eosinophilia in recent adult immigrants from Africa; the diagnostic usefulness of parasitologic and serologic tests; and the relationship between specific helminthic infections, country of origin, and degree of eosinophilia. After they gave written consent, 788 African immigrants were screened by examination of detailed medical records, physical examination, routine laboratory tests, serologic tests, the Mantoux test, and chest radiographs. Of the immigrants, 213 met the following inclusion criteria: 1) arrival within 6 months; 2) age >18 years, and 3) eosinophilia (>0.45×109 eosinophils/L). Direct parasitologic tests included the examination of 3 stool samples (both Kato-Katz and Ritchie techniques were used for each sample) and specific tests for Strongyloides stercoralis (Baermann test and agar culture) (1), optic microscopy of a terminal urine specimen, and Knotts test for microfilaremia. The immune chromatographic test for Wuchereria bancrofti (ICT Filariasis Binax, Portland, ME, USA), skin snips, and the Mazotti test were also used in selected cases.
ELISAs with crude extracts of adult Dirofilaria immitis adult worm antigens (AWA Di) (2), Schistosoma bovis worm antigens (3), Fasciola hepatica excretory/secretory antigens (4), and Trichinella spiralis L1 antigens (5) were used. Polystyrene microtiter plates were coated with 100 μL antigens per well in carbonate buffer (pH 9.6). Serum diluted 1:100 was added and incubated for 1 h at 37°C. Horseradish peroxidase goat anti-human immunoglobulin G (Sigma, Saint Louis, MO, USA) was added at different dilutions. Washes were performed 3 times with 200 μL phosphate-buffered saline–Tween 20 per well. After incubation for 1 h at 37°C, the substrate solution (ortho-phenylenediamine-H2O2) was added, and the reaction was stopped with 3N H2SO4.
Assay sensitivities were evaluated by using serum specimens from patients with a definite diagnosis of isolated helminthic disease (Table 1). In all patients, adequate parasitologic tests showed no other helminthic infection. To evaluate specificities, we used serum samples from Spanish blood donors; samples from healthy controls from sub-Saharan Africa; and samples from patients with isolated helminthic, protozoal, bacterial, or viral infections (Table 1). Healthy controls from sub-Saharan Africa were clinically evaluated; they did not have eosinophilia, and results of a systematic investigation for helminthic infections (using stool samples, urine samples, and Knotts test) were negative.
Moreover, an ELISA was used to test for strongyloidiasis with somatic larvae antigens from Strongyloides venezuelensis. Although the ELISA is 100% sensitive, its low specificity precluded its use as a diagnostic tool.
The SPSS 11.5 statistical package (available from http://www.spss.com) was used for analyses. The level of significance accepted was <0.05, and results were expressed as means plus standard deviation (SD). The receiver-operating-characteristic curve was used to establish ELISA cut-offs. The χ2 and the Fisher exact tests were used to evaluate the association between demographic variables and final diagnoses, and the Student t test was used to compare the degree of eosinophilia among patients with single and multiple infections. Analysis of variance and post-hoc tests were used to compare the mean eosinophil counts in each final diagnosis.
We found that 213 (27.0%) of 788 immigrants whose conditions were analyzed had eosinophilia. Of these, 191 (89.7%) were male, with a mean age of 27.4 years (SD 8.3). Two hundred two (94.9%) patients were from sub-Saharan countries, mainly Nigeria (24.1%), Sierra Leone (17.3%), Ghana (15.0%), and Mali (8.9%); 165 (77.1%) patients had 0.450–0.999×109 eosinophils/μL, 47 (21.9%) had 1.000–2.999×109 eosinophils/μL, and 1 patient had >3.000×109 eosinophils/μL.
One hundred fifty-four study participants (72.3%) were asymptomatic. In symptomatic patients (28.0%), the most frequent clinical features were lymphadenopathy (6.1%), pruritus (5.6%), and skin lesions (3.3%).
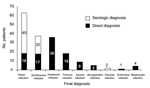
A final diagnosis was made in 161 cases (75.6%): 116 (54.5%) had 1 parasite, 30 (14.1%) had 2, and 15 (7.0%) had >3. The most frequent parasites were filariae (n = 63, 29.6%), schistosomes (n = 37, 17.4%), hookworms (n = 36, 16.8%), and Trichuris spp. (n = 18, 8.4%) (Figure 1). Direct methods were used in 60 (37.2%) patients, indirect methods were used in 80 (49.6%), and both methods in 21 (13.0%) patients. Stool microscopy and filarial and schistosomal serologic testing yielded the highest positive result rates (Table 2). The country of origin was statistically associated (p<0.05) with the final diagnosis: 77% of the patients with eosinophilia from Cameroon had filariasis, 63% of the patients from Mali had schistosomiasis, and 30.8% of the patients from Nigeria had hookworm infection.
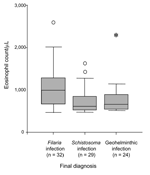
The mean eosinophil count was significantly higher in patients with a final diagnosis than in those whose conditions were not diagnosed (871 ± 431 vs. 643 ± 179) (p<0.05), and the mean count was higher also in patients with 2 or more parasites than in patients with 1 (1,045 ± 641 vs. 827 ± 389) (p<0.05). Among patients with 1 helminthic disease, those with filariasis had higher eosinophil counts than those with schistosomiasis or geohelminthic infection (p<0.05) (Figure 2).
Eosinophilia is frequent in travelers and expatriates from tropical areas (6–12). However, its prevalence is variable (3.1%–50%), depending on the population studied (more frequent in immigrants than in travelers), the areas where infection occurs (mainly sub-Saharan Africa or Southeast Asia), and the design of the study (prospective or retrospective). In this prospective work, we studied a homogeneous population of immigrants who had recently arrived from Africa, and we detected eosinophilia in 27%.
Studies of persons with imported eosinophilia have made a diagnosis that identified the etiologic agent in 15% to 64% of cases (depending on the population, the selected eosinophil count, and the methods) (6–13). Using direct and serologic methods (10,13), we detected helminthic infections in 75% of the patients. In all series, the main diagnoses are filarial, schistosomal, and geohelminthic infections. Only 27.7% of our patients had related signs or symptoms, which indicates that a proper investigation can detect many asymptomatic infections.
The sensitivities of our serologic tests were >90%, with specificities of 85%–97%. Using D. immitis antigens for the immunodiagnosis of tropical filariasis (14), we obtained a sensitivity of 90% for microfilaremia, with 97% specificity. The utility of adult worm antigens of S. bovis for serodiagnosis of schistosomiasis has been recently demonstrated (3).
Our high diagnostic yield with filarial (30%) and schistosomal (28%) serologic testing is similar to that obtained by Whetham et al. in travelers returning from West Africa (10). Among the direct methods, stool microscopy was the most sensitive (35%). However, serologic testing detected another parasitic infection (mainly filarial or schistosomal) when direct tests showed only a geohelminthic infection (13.2%), which suggests that direct and indirect tests are complementary in this population.
The proportion of Strongyloides spp. infection diagnosed was lower than in almost all other similar studies (6–12) because we could not ascertain it by stool positivity only, because of the low specificity of Strongyloides serologic testing available to us. Patients from Mali with eosinophilia had schistosomiasis more frequently, as reported in some European studies (15). However, we found a significant correlation between filarial or hookworm infection and immigration from Cameroon and Nigeria, respectively, an association not described previously. Finally, filariasis induces higher eosinophil counts than other parasitic infections, likely because the parasite inhabits blood and tissue and is not limited to the gut lumen. Our results show that 1) eosinophilia is frequent in recently arrived African immigrants, 2) helminthic infections can be diagnosed by using both parasitologic and serologic tests, 3) an immigrant's country of origin may suggest specific parasitic diseases, and 4) higher eosinophil counts usually indicate filariasis.
Dr Pardo is a specialist in internal medicine at Hospital Clínico Universitario de Salamanca (Spain). His research interests focus on diseases imported by travelers and immigrants.
Acknowledgments
We thank G.H. Jenkins for his help with the English version of the manuscript.
This work has been supported in part by grants from the Fondo de Investigación Sanitaria (FIS reference no. 01/0685), Comunidad Canaria (PI 2003/024), and the Red de Centros de Investigación en Medicina Tropical (FIS reference nos. C03/04 and PI 05552).
References
- Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:1040–7. DOIPubMedGoogle Scholar
- Perera L, Pérez Arellano JL, Cordero M, Simón F, Muro A. Utility of antibodies against a 22 kDa molecule of Dirofilaria immitis in the diagnosis of human pulmonary dirofilariosis. Trop Med Int Health. 1998;3:151–5. DOIPubMedGoogle Scholar
- Pardo J, Carranza C, Turrientes MC, Pérez Arellano JL, Lopez-Velez R, Ramajo V. Utility of Schistosoma bovis adult worm antigens for diagnosis of human schistosomiasis by enzyme-linked immunosorbent assay and electroimmuno-transfer blot techniques. Clin Diagn Lab Immunol. 2004;11:1165–70.PubMedGoogle Scholar
- Hillyer GV, Soler de Galanes M. Identification of a 17-kDa Fasciola hepatica immunodiagnostic antigen by enzyme-linked immunoelectrotransfer blot technique. J Clin Microbiol. 1988;26:2048–53.PubMedGoogle Scholar
- Alcántara P, Correa D. Human humoral immune response against Trichinella spiralis. Int J Parasitol. 1993;23:657–60. DOIPubMedGoogle Scholar
- Harries AD, Myers B, Bhattacharrya D. Eosinophilia in Caucasians returning from the tropics. Trans R Soc Trop Med Hyg. 1986;80:327–8. DOIPubMedGoogle Scholar
- Nutman TB, Ottesen EA, Ieng S, Samuels J, Kimball E, Lutkoski M, Eosinophilia in Southeast Asian refugees: evaluation at a referral center. J Infect Dis. 1987;155:309–13. DOIPubMedGoogle Scholar
- Libman MD, MacLean JD, Gyorkos TW. Screening for schistosomiasis, filariasis, and strongyloidiasis among expatriates returning from the tropics. Clin Infect Dis. 1993;17:353–9. DOIPubMedGoogle Scholar
- Schulte C, Krebs B, Jelinek T, Nothdurft HD, von Sonnenburg F, Loscher T. Diagnostic significance of blood eosinophilia in returning travelers. Clin Infect Dis. 2002;34:407–11. DOIPubMedGoogle Scholar
- Whetham J, Day JN, Armstrong M, Chiodini PL, Whitty CJ. Investigation of tropical eosinophilia; assessing a strategy based on geographical area. J Infect. 2003;46:180–5. DOIPubMedGoogle Scholar
- Lopez-Velez R, Huerga H, Turrientes MC. Infectious diseases in immigrants from the perspective of a tropical medicine referral unit. Am J Trop Med Hyg. 2003;69:115–21.PubMedGoogle Scholar
- Seybolt LM, Christiansen D, Barnett ED. Diagnostic evaluation of newly arrived asymptomatic refugees with eosinophilia. Clin Infect Dis. 2006;42:363–7. DOIPubMedGoogle Scholar
- Whitty CJ, Carroll B, Armstrong M, Dow C, Snashall D, Marshall T, Utility of history, examination and laboratory tests in screening those returning to Europe from the tropics for parasitic infection. Trop Med Int Health. 2000;5:818–23. DOIPubMedGoogle Scholar
- Dumenigo Ripoll B, Espino Hernandez AM, Menendez Valonga MC, Finlay Villalvilla C. Excretion-secretion antigens from adult Dirofilaria immitis in the diagnosis of human filariasis by solid phase immunoenzyme assay. Rev Cubana Med Trop. 1991;43:162–6.PubMedGoogle Scholar
- Grobusch MP, Muhlberger N, Jelinek T, Bisoffi Z, Corachan M, Harms G, Imported schistosomiasis in Europe: sentinel surveillance data from TropNetEurop. J Travel Med. 2003;10:164–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 12, Number 10—October 2006
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
José Luis Pérez-Arellano, Departamento de Ciencias Médicas y Quirúrgicas, Centro de Ciencias de la Salud, Universidad de las Palmas de Gran Canaria, 35080 Las Palmas de Gran Canaria, Spain
Top