Volume 29, Number 11—November 2023
Dispatch
Genetic Characterization of Extensively Drug-Resistant Shigella sonnei Infections, Spain, 2021–2022
Abstract
In 2022, the United Kingdom reported an increase in drug resistance in Shigella sonnei isolates. We report 33 cases in Spain genetically related to the UK cases and 4 cases with similar antimicrobial resistance profiles infected with genetically distant strains. Our results suggest circulation of multiple genetic clusters of multidrug-resistant S. sonnei in Spain.
On January 27, 2022, the United Kingdom reported an increased number of infections with extensively drug-resistant (XDR) Shigella sonnei during September 1, 2021–January 10, 2022 (1). A total of 146 cases were later reported in 9 other countries in Europe, all having either a similar XDR profile or close genetic relationship to the UK cases (2). Most cases were linked to sexual transmission between gay, bisexual, and other men who have sex with men (MSM) (2).
In Spain, information on S. sonnei exposure is rarely available (only in 7.2% of cases), but person-to-person transmission is the most frequently observed. Previous studies in Spain reported circulation of lineages of S. sonnei resistant to first- and second-line oral treatments among MSM in different autonomous communities (Catalunya, Andalucía, País Vasco, and Madrid) as early as 2015 (3–7). We describe the multidrug-resistant (MDR) isolates of S. sonnei circulating in Spain during January 2021–April 2022.
For this investigation, we defined a suspected case as a patient with laboratory-confirmed S. sonnei infection; an MDR profile characterized by nonsusceptibility to >1 agent in >3 of the antimicrobial categories tested, including third-generation cephalosporins, aminoglycosides, sulfamethoxazole, and fluroquinolones; and a specimen collected in Spain during January 1, 2021–April 1, 2022. Over the study period, hospitals voluntarily sent 51 S. sonnei isolates to the National Center of Microbiology in Spain, and epidemiologic data were collected from laboratory request forms or by directly contacting the hospitals. From those cases, we identified 37 (72%) suspected cases across 12 autonomous communities (Appendix 1). This finding represented a dramatic increase compared with 2019 data from Spain, in which only 6% of isolates tested in the National Center of Microbiology were identified as MDR (C. Jacqueline et al., unpub. data). We excluded 2020 from review because of the COVID-19 pandemic.
The median age of the persons with suspected cases was 34 (range 18–75) years (Appendix 2). Seventeen (46%) persons reported diarrhea, and 1 person was asymptomatic (tested as contact of another case); for 19 (51%) persons, symptoms were not reported. Eight (22%) persons were hospitalized for fever, enterocolitis, pancolitis, and dehydration; hospitalization status was unknown for 2 (5%) persons. The percentage of hospitalizations was similar to data from 2018 (20%) but higher than data from 2019 (11%). Mean duration of hospitalization was 5.7 (range 2–10) days, and no person died from their infection. When considered appropriate, patients were treated with ertapenem, meropenem, ciprofloxacin, amoxicillin/clavulanic acid, metronidazole, or fosfomycin (alone or in combination). Antibiotic treatment failure was occasionally observed and resolved by a change in treatment (7).
Nineteen (51%) persons from 7 distinct autonomous communities were identified as MSM, and sexual transmission was hypothesized in 4 groups of sexual partners. One person reported sexual contact with persons in France. No person reported exposure to potentially contaminated food or water. Some patients were HIV positive (frequency is omitted to prevent deductive disclosure), and only 2 persons reported using preexposure prophylaxis (18 cases reported not using preexposure prophylaxis; information was unavailable for the 17 other cases).
We performed whole-genome sequencing (WGS; Illumina Inc., https://www.illumina.com) on all isolates from suspected cases. Sequences can be accessed on Enterobase (https://enterobase.warwick.ac.uk; uberstrains ESC_BB1296AA to ESC_BB1332AA). Using the Escherichia/Shigella scheme of Enterobase, we performed a core genome multilocus sequence typing analysis. We found 33 (89%) isolates that were within 7 allelic differences (absent alleles were not considered as differences) of the 3 representative outbreak sequences shared by the United Kingdom in the EpiPulse event notification portal (https://www.ecdc.europa.eu/en/publications-data/epipulse-european-surveillance-portal-infectious-diseases). We defined all isolates belonging to that genetic cluster as confirmed cases and belonging to sequence type (ST) 152. Two ST152 isolates showed a high number of allelic differences compared with the main cluster, including 1 isolate from a female case-patient. In addition, we identified 2 isolates as belonging to different sequence types, ST3075 and ST5390 (Appendix 1).
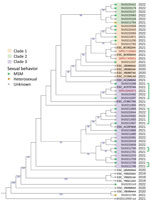
We investigated the genetic diversity of ST152 isolates using a single-nucleotide polymorphism analysis (Center for Genomic Epidemiology, https://cge.food.dtu.dk/services/CSIPhylogeny). We included all the sequences available on Enterobase that corresponded to S. sonnei ST152 cases in Spain during 2019–2022. We found a low genetic diversity overall, especially between the sequences of confirmed cases, but we observed 3 phylogenetical clades (confirmed by hierBAPS, https://github.com/gtonkinhill/rhierbaps) (Figure). We observed 1–9 single-nucleotide polymorphisms of difference between cases where sexual transmission was hypothesized.
We identified the genetic determinants of resistance using PlasmidFinder 2.1 (8) and ResFinder 4.1 (9,10). First, we found that all confirmed cases harbored the plasmid replicon IncFII, which carried the gene blaCTX-M-27, responsible for resistance to ampicillin, cefepime, cefotaxime, and ceftazidime. However, the ST152 isolate from the female case-patient and the ST3075 and ST5390 isolates harbored different extended-spectrum β-lactamase–producing genes (Appendix Table). Resistance to streptomycin was associated with aadA5, resistance to sulfamethoxazole was associated with sul1, and resistance to trimethoprim was associated with dfrA1, all of which were also harbored by the IncFII plasmid. The gene mphA conferring resistance to azithromycin was present in all but 2 isolates. We also detected an additional plasmid replicon, IncB/O/K/Z, in 30 isolates. In the core genome, the same point mutation S83L in the gyrA gene, conferring fluoroquinolone resistance, was present in all isolates.
We describe the circulation in Spain of a cluster of extended spectrum β-lactamase–producing and MDR S. sonnei infections genetically related with those observed in a contemporaneous UK outbreak. Because most of the isolates harbored the gene conferring azithromycin resistance, we hypothesize that they would be XDR, even though we did not confirm it phenotypically. We also identified strains of MDR S. sonnei that belonged to STs other than the one described in the United Kingdom. This finding raises concerns about the ability to manage the spread of MDR and XDR Shigella infections and highlights the need to strengthen surveillance of shigellosis.
In the United Kingdom, sexual transmission between MSM was identified as the main factor of circulation for the strains harboring the IncFII plasmid replicon (1). Although we cannot exclude other confounding factors, our results point in the same direction. Indeed, food exposure was not reported, and 19 confirmed case-patients were MSM, even though the respondent rate for sexual orientation was low in our study. However, identifying a female case-patient and a heterosexual male case-patient suggests circulation of MDR S. sonnei outside of the MSM population. In Spain, efforts should be made to obtain information related to exposure and to recommend the use of microbiological culture and WGS to identify chains of transmission and antibiotic resistance. Such efforts will be crucial in preventing further selection of antimicrobial resistance, avoiding possible treatment failures, and managing what might become a global outbreak.
Further monitoring of the situation in Spain, as well as in Europe, will be necessary to assess the extent of the circulation of XDR S. sonnei. Although more studies are needed to confirm the role of sexual transmission in Spain, communication campaigns, notably in HIV and preexposure prophylaxis clinics, could inform MSM on ways to minimize the risk of infection. Finally, alerting healthcare professionals to the role of sexual transmission in S. sonnei infections is critical for obtaining information on sexual history and identifying new cases, particularly in adult men with acute diarrhea.
Dr. Jacqueline is a EUPHEM fellow (European Centre for Disease Prevention and Control, Stockholm, Sweden) working at the Instituto de Salud Carlos III in Madrid. Her primary research interests are public health microbiology, epidemiology, and outbreak investigation.
Acknowledgments
We thank the Genomic and Bioinformatic departments of the National Center of Microbiology for their help with WGS and Kristina Zugazaga from the Hospital of Basurto for her technical support.
Financial support for this work came from the National Institute of Health Carlos III with the project Acción Estratégica de Salud Intramural (AESI; PI21CIII/00029).
References
- Charles H, Prochazka M, Thorley K, Crewdson A, Greig DR, Jenkins C, et al.; Outbreak Control Team. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021-22: a descriptive epidemiological study. Lancet Infect Dis. 2022;22:1503–10. DOIPubMedGoogle Scholar
- European Centre for Disease Prevention and Control. Increase in extensively-drug resistant Shigella sonnei infections in men who have sex with men in the EU/EEA and the UK—23 February 2022. Stockholm: The Centre; 2022.
- Moreno-Mingorance A, Espinal P, Rodriguez V, Goterris L, Fàbrega A, Serra-Pladevall J, et al. Circulation of multi-drug-resistant Shigella sonnei and Shigella flexneri among men who have sex with men in Barcelona, Spain, 2015-2019. Int J Antimicrob Agents. 2021;58:
106378 . DOIPubMedGoogle Scholar - Ortiz de la Rosa JM, Rodríguez-Villodres Á, Casimiro-Soriguer CS, Ruiz-Pérez De Pipaón M, Briones E, Aznar Fernández M, et al. MDR Shigella sonnei in Spain: an ever-evolving emerging threat? JAC Antimicrob Resist. 2022;4:dlac090.
- González Donapetry P, Pescador Martín P, Gómez-Gil Mira R, Ruiz Carrascoso G. Imported infection by CTX-M-15 extended-spectrum beta-lactamase-producing Shigella sonnei. [Engl Ed]. Enferm Infecc Microbiol Clin (Engl Ed). 2019;37:141. DOIPubMedGoogle Scholar
- López-Cerero L, Stolz E, Pulido MR, Pascual A. Characterization of extended-spectrum ß-lactamase-producing Shigella sonnei in Spain: expanding the geographic distribution of sequence type 152/CTX-M-27 clone. Antimicrob Agents Chemother. 2022;66:
e0033422 . DOIPubMedGoogle Scholar - Fernández Vecilla D, Zugazaga Inchaurza K, Lombide Aguirre I, Díaz de Tuesta Del Arco JL. Phenotypic and genotypic characterization of Shigella sonnei carrying the extended-spectrum beta-lactamase CTX-M-27. A report of two cases in Spain in men who have sex with men. [Engl Ed]. Enferm Infecc Microbiol Clin (Engl Ed). 2023;41:248–50. DOIPubMedGoogle Scholar
- Carattoli A, Zankari E, García-fernández A, Larsen V, Lund O, Villa L, Aarestrup M, Hasman H. In silico detection and typing of plasmids using Plasmidfinder and plasmid multilocus sequence typing. 2014;58:3895–903.
- Bortolaia V, Kaas RS, Ruppe E, Roberts MC, Schwarz S, Cattoir V, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75:3491–500. DOIPubMedGoogle Scholar
- Zankari E, Allesøe R, Joensen KG, Cavaco LM, Lund O, Aarestrup FM. PointFinder: a novel web tool for WGS-based detection of antimicrobial resistance associated with chromosomal point mutations in bacterial pathogens. J Antimicrob Chemother. 2017;72:2764–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: October 20, 2023
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|