Volume 29, Number 11—November 2023
Research
Risk Factors for Recent HIV Infections among Adults in 14 Countries in Africa Identified by Population-Based HIV Impact Assessment Surveys, 2015–2019
Abstract
Identifying persons who have newly acquired HIV infections is critical for characterizing the HIV epidemic direction. We analyzed pooled data from nationally representative Population-Based HIV Impact Assessment surveys conducted across 14 countries in Africa for recent infection risk factors. We included adults 15–49 years of age who had sex during the previous year and used a recent infection testing algorithm to distinguish recent from long-term infections. We collected risk factor information via participant interviews and assessed correlates of recent infection using multinomial logistic regression, incorporating each surveyʼs complex sampling design. Compared with HIV-negative persons, persons with higher odds of recent HIV infection were women, were divorced/separated/widowed, had multiple recent sex partners, had a recent HIV-positive sex partner or one with unknown status, and lived in communities with higher HIV viremia prevalence. Prevention programs focusing on persons at higher risk for HIV and their sexual partners will contribute to reducing HIV incidence.
Sub-Saharan Africa has the highest HIV infection incidence and prevalence in the world (1,2). Although incidence is declining (3), more progress is needed to reduce transmission to a sufficient level that achieves global epidemic control. Several metrics for epidemic control have been proposed, including an incidence:mortality ratio (number of new HIV infections:total number of deaths from all causes among HIV-infected persons), a metric used providing that both new infections and deaths are low and declining (4). In sub-Saharan Africa, the Joint United Nations Programme on HIV/AIDS (UNAIDS) estimates that women and girls accounted for 63% of all new HIV infections in 2021 (2). Determining risk factors for HIV acquisition in countries with generalized HIV epidemics can help identify appropriate groups for tailored prevention programming and can support testing for those at highest risk of acquiring infection.
Methods used to examine risk factors for HIV often compare HIV-negative and HIV-positive persons (prevalence) (5–7) or use longitudinal cohort studies that comprise HIV-negative persons at baseline (8–11). Each of those methods has drawbacks. The prevalence approach does not distinguish recent from long-term infections, making it difficult to determine whether risk factors preceded the infection (12). In addition, risk factors for HIV might change over time, and programmatic efforts need to assess who is at the highest risk of acquiring new infections to prevent transmission. Longitudinal cohorts can establish timing of infection; however, they require long follow-up periods and large sample sizes and are subject to attrition bias that might not be equal across risk factors (12).
Assays that distinguish recent from long-term HIV-1 infections present an opportunity to estimate HIV incidence and assess risk factors in cross-sectional population-based household surveys (12,13). The limiting-antigen (LAg) avidity enzyme immunoassay (EIA) has been well characterized, validated, and used for the detection of recent infections and estimation of HIV-1 incidence as part of a recent infection testing algorithm (RITA) in cross-sectional surveys, including Population-Based HIV Impact Assessment (PHIA) surveys (14,15). Therefore, PHIA surveys can identify risk factors for new HIV infections in the general population across multiple countries in sub-Saharan Africa by using the largest sample of recent infections. We identified demographic and behavioral risk factors for recent HIV infections among sexually active adults across 14 sub-Saharan Africa countries and assessed whether those factors differed between recent and long-term infections.
Study Design
PHIAs are nationally representative, cross-sectional, population-based surveys of households across each country (16,17). A 2-stage, stratified cluster sample design was used in each survey: enumeration areas were selected within strata (subnational units, such as regions) by using a probability proportional to size method, and households within enumeration areas were randomly selected in the second stage. Weights were calculated to account for unequal probability of household selection, nonresponse, and noncoverage. Within selected households, the household head completed a household survey, and eligible household members completed individual interviews and had blood collected after providing consent for each survey component.
Study Population
We used data from PHIA surveys completed in 14 countries during 2015–2019: Cameroon, Cote d’Ivoire, Eswatini, Ethiopia, Kenya, Lesotho, Malawi, Namibia, Nigeria, Rwanda, Tanzania, Uganda, Zambia, and Zimbabwe. We pooled data across PHIAs in a multicountry analysis because of small sample sizes for recent infections within each country. We included adults 15–49 years of age who reported engaging in sexual activity during the year before their interview. We only included persons who consented to a blood draw and had a valid final RITA classification.
Variable Definitions
We explored demographic and behavioral variables collected during participant interviews to identify potential risk factors. Demographic factors were country, sex, age, marital status, education, and household wealth. Behavioral factors were number of recent sexual partners (during the previous 12 months), age of sexual debut, HIV status of partner(s), age of partner(s), condom usage, and voluntary medical male circumcision status. Age of sexual debut was divided into <18 or >18 years categories; 18 years of age was the median. The numbers of partners in the previous year were grouped into categories (0, 1, or >2 partners), consistent with previous literature and an examination of the data (12,13). Age groups were 15–24, 25–34, and 35–49 years, according to published precedent.
We calculated community viremia levels within each stratum in each country. We defined participants with long-term HIV infections and detectable viral load of >1,000 copies/mL as viremic and all HIV-negative or HIV-positive participants with an undetectable viral load as nonviremic. We excluded persons with recent HIV infection (as defined in the next paragraph) from community viremia calculations, which were calculated as the weighted number of viremic persons divided by the weighted number of viremic plus nonviremic persons within each stratum. We then categorized each stratum into quantiles representing the percentages of persons with nonsuppressed HIV infection within the stratum.
The 3 primary outcome categories for each participant were recent HIV infection, long-term HIV infection, or HIV negative. All participants were tested for HIV in the household according to each country’s national testing algorithm. Confirmatory HIV testing was completed in all countries except Uganda by using the Geenius HIV-1/2 rapid test (Bio-Rad Laboratories, https://www.bio-rad.com). We classified HIV according to confirmatory testing and excluded a small number of participants (n<25) who tested positive for HIV-2 but not HIV-1 from recency testing because the LAg-Avidity EIA is meant for HIV-1 recency classification only. Among HIV-positive participants, RITA was used to distinguish recent from long-term infections. The first step of RITA used the LAg-Avidity EIA (Sedia Biosciences Corp., https://www.sediabio.com, for plasma specimens or Maxim Biomedical, https://www.maximbio.com, for dried blood spot specimens), which assesses development of antibody avidity. We classified participants with a median normalized optical density <1.5 for plasma samples (or <1.0 for dried blood spot samples where venous blood could not be collected [<5% of participants]) as LAg-recent infections. Next, we categorized participants as recently infected if they had LAg-recent infections, HIV viral loads >1,000 copies/mL, and an absence of antiretroviral drug metabolites in their blood by using RITA (14,15). All HIV-1–positive participants who did not meet the criteria for recent infection were categorized as having long-term HIV infection.
Statistical Analysis
Initial data analysis strategies were bivariate comparisons between HIV-negative participants and those who had a recent HIV infection or long-term HIV infections. We calculated an overall χ2 test statistic and unadjusted odds ratios for categorical variables. We used Taylor series weights and variables representing strata and units for all analyses. We performed analyses by using SAS version 9.4 (SAS Institute, https://www.sas.com) and considered p values <0.05 statistically significant.
Our model-building strategy incorporated candidate exposure variables with bivariate p values <0.20. In cases where variables were colinear, we included only 1 variable or set of variables in the final model. Only variables collected consistently across countries were eligible for the multivariable model. We used multinomial logistic regression to calculate adjusted weighted odds ratios accounting for the complex survey sample design.
Ethics Statement
PHIA surveys were funded by the US President’s Emergency Plan for AIDS Relief (PEPFAR) (18); technical assistance was provided by the US Centers for Disease Control and Prevention. The surveys were conducted through cooperative agreements with grantees/federal entities, including country Ministries of Health, ICAP at Columbia University (New York, New York, USA), and the University of Maryland (Baltimore, MD, USA). Each survey was approved by human subject institutional review boards specific for each country, cooperative agreement grantees/federal entities conducting the survey, or the US Centers for Disease Control and Prevention.
Population Description
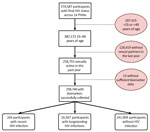
Across the 14 countries included in this analysis, we identified 16,831 HIV-positive and 241,909 HIV-negative PHIA participants (Figure). Of the 16,831 HIV-positive participants, 264 (1.6% of all HIV-positive participants) had recent infections and 16,567 had long-term infections. Sample sizes of participants who met inclusion criteria ranged from 5,874 in Eswatini to 95,463 in Nigeria (Table 1). Biomarker (blood draw) response rates ranged from 86.7% in Malawi to 99.0% in Uganda for female participants and 85.3% in Namibia to 98.5% in Uganda for male participants.
Bivariate Analysis Findings
We performed weighted bivariate comparisons between HIV-negative and recently infected participants (Table 2). Region was significantly associated with recent HIV infection; persons living in western Africa represented only 27.1% of persons with recent HIV infections but represented 51.5% of those who were HIV negative. In addition, female sex, age 25–34 years, and divorced or separated marital status were associated with recent HIV infection. Among young adults 15–24 years of age, female participants accounted for 72.1% of recent infections. Demographic variables not associated with recent infection were urban and rural locations, household wealth, and working during the previous 12 months.
Behavioral factors were also associated with the prevalence of recent infections. For example, compared with HIV-negative participants, those who had recent HIV infections were more likely to have had a sexual debut at <18 years of age, had >1 partner in the previous 12 months, had sex with a partner with whom they were not living, not used condoms during their last sexual intercourse with a nonregular partner, and had sex within the previous 12 months with a partner who had unknown HIV status or an HIV-positive status (Table 2).
We also performed weighted bivariate comparisons of recently infected participants and those with long-term infections (Table 2). Compared with participants 35–49 years of age, adolescents and young adults (15–34 years of age) had a greater percentage of recent infections than long-term infections. Participants who were never married or were divorced/separated had a greater percentage of recent than long-term infections, whereas those who were widowed were more likely to have long-term infections. Sex, employment, education, household wealth, and urbanicity were not associated with recent versus long-term infections. Behavioral factors more common in participants who had recent HIV infections (compared with those with long-term HIV infections) were sexual debut at <18 years of age, >1 sexual partner in the previous 12 months, having partners other than a spouse or live-in partner in the previous 12 months, not using a condom during their last sexual intercourse, and having a partner in the previous 12 months who had unknown HIV status (Table 2).
We performed bivariate exploratory comparisons of variables that were not collected consistently across all PHIAs or were only relevant to population subgroups (Table 3). Participants who had a sexually transmitted disease diagnosis or sexually transmitted infection symptoms and male participants who did not have a medical circumcision were more likely to be recently infected than HIV-negative. In addition, participants who had long-term infections were more likely to have previously been tested for HIV >1 year ago and were more likely to be uncircumcised than those who had a recent HIV infection (Table 3).
Multivariate Analysis Findings
In the adjusted model, the southern, southeastern, and eastern Africa regions and community-level viremia remained significantly associated with recent infections (Table 4). Participants living in countries within eastern, southeastern, and southern Africa had higher odds of recent HIV infection compared with those in western Africa, even after adjusting for community-level viremia. In addition, participants grouped in the third and fourth highest quartiles of community-level viremia were more likely to have a recent HIV infection than those in the lowest quartile; the odds of recent infection increased with each viremia quartile.
Individual demographic characteristics, including sex and marital status, remained significantly associated with HIV acquisition; female participants had 1.82 times greater odds of recent HIV infection than for male participants. In addition, participants who were divorced, separated, or widowed had 3.58 times greater odds of recent HIV infection than for those who were never married. Age group was not significantly associated with HIV acquisition risk in adjusted models (Table 4). Sexual behavior characteristics that remained significantly associated with recent HIV acquisition were having >1 partner in the previous 12 months (adjusted odds ratio [aOR] 1.92) and having >1 partner who thought or was told they were HIV-positive (aOR 7.25) or had unknown HIV status (aOR 2.05).
In the multivariable model, compared with those with longstanding infections, those 15–24 years of age (aOR 4.79) and 25–34 years of age (aOR 2.23) were more likely to have a recently acquired infection than were participants who were 35–49 years of age (Table 5). Participants who used a condom the last time they had sex (aOR 0.46) and had >1 partner in the previous 12 months that the participant believed or knew to be HIV-positive (aOR 0.17) were less likely to have a recent infection than a long-term infection. Region, sex, marital status, age of sexual debut, partner age differences, and community-level viremia did not significantly differ between those with recent and long-term infections.
We compared persons recently infected with HIV, HIV-negative persons, and persons with long-term HIV infections in 14 countries within sub-Saharan Africa by using large, nationally representative, population-based surveys. Participants living in regions of sub-Saharan Africa with higher HIV prevalence were considerably more likely to have a recent HIV infection. Similarly, participants living in communities with higher prevalence of HIV viremia had higher odds of recent HIV infection. Both associations persisted after controlling for individual risk factors. Treatment as prevention (TasP) is a primary strategy for ending the HIV epidemic and is an essential strategy within the conceptual framework of UNAIDS 95–95–95 goals (that 95% of people living with HIV/AIDS know their status, 95% of those who know their status are on treatment, and 95% of those on treatment are virally suppressed). Multiple studies have shown that persons with undetectable levels of HIV (i.e., HIV viral load <200 copies/mL blood) have essentially no risk of transmitting HIV through sex (19,20). However, population-based studies that assess the effects of TasP on HIV incidence in communities have had fewer clear outcomes; some studies found a lack of population-level effect of TasP (21). Persons living in areas with higher HIV prevalence or higher prevalence of viremic people living with HIV/AIDS have increased likelihood of acquiring HIV infections (22). This finding reinforces the potential benefits of identifying persons who are unaware of their HIV infection and enrolling them in treatment programs to achieve sustained HIV viral suppression, as well as benefits of prevention interventions, such as scale-up of HIV preexposure prophylaxis according to programmatic need.
Consistent with existing literature from sub-Saharan Africa, we found that recent HIV infection was higher in female than male participants; female participants accounted for 65% of recent infections and had ≈2 times the adjusted odds of recent HIV infection compared with male participants (12,23). The estimated proportion of new infections occurring among adolescents and young adults 15–24 years of age (31.4%) was largely consistent with UNAIDS estimates of 2 in 7 new infections occurring among that age group (24). We also found that >70% of new HIV infections among adolescents and young adults were acquired by female participants, in line with the 60%–80% estimates by UNAIDS (25). Those findings highlight the continued need for HIV prevention programs for women and girls, along with other sexual and reproductive health services. Gender disparities in HIV incidence also highlight the need to engage male patients in treatment uptake and retention efforts to further reduce infections among their female partners (26). Age group was not associated with risk for recent HIV infection in adjusted models, indicating the importance of HIV prevention programming across the age continuum among sexually active adolescents and adults.
Compared with participants having sex only with partners they believed or knew were negative for HIV, having a partner that the participant knew or believed was positive for HIV or a partner with unknown HIV status was associated with acquiring a recent HIV infection. The relationship was stronger for those with a partner known or believed to be positive for HIV. Still, most new infections occurred among those who had a sex partner with unknown HIV status. Very few participants with recent HIV infection had sex only with partners they thought were negative for HIV. Having sex with a partner outside of marriage, a sexual debut before turning 18 years of age, and having multiple partners were also associated with an increased risk for new HIV infection. Those findings are not surprising, because they are broadly consistent with similar analyses completed over the previous 2 decades (12,13). Although HIV incidence has decreased during that period, further declines in incidence and reduction in incidence disparities might rely on continued targeted testing to identify those persons at risk of transmitting HIV, as well as interventions that encourage disclosure of positive status, promote access to antiretroviral therapy to suppress viral load, prevent transmission, reduce the number of sexual partners, and promote safe sex and access to preexposure prophylaxis.
Certain factors could not be included in the final model because those data were collected inconsistently across countries or were applicable only to subpopulations. However, voluntary medical male circumcision has been shown to reduce the risk of HIV acquisition by 38%–66% (27), and our bivariate comparisons of participants with recent infections compared with HIV-negative participants are consistent with those data. Additional analyses of PHIA data from fewer countries but using methods designed to specifically determine the effect of male medical circumcision on HIV incidence have similarly found a substantial protective effect, particularly among younger men and boys 15–34 years of age (28).
Although using RITAs in cross-sectional surveys enables the examination of risk factors for new infections, limitations to this approach exist. PHIAs are designed to estimate national incidence rates by using RITAs and are not powered to examine any specific associations between potential risk factors and recent infections. Even though PHIAs were used in some countries with the highest HIV prevalence worldwide and sample sizes were large, very few (range 4–31) recent infections among the study populations were identified. The rarity of the outcome precluded country-specific analyses of risk factors for recent infections. In addition, cultural context, epidemic dynamics, and responses of governments to the epidemic are not homogenous across the continent. Therefore, we could not examine those nuances across or within countries. The size and scope of PHIAs prevents data being available in near real-time; thus, delays in monitoring HIV-acquisition trends using survey-based approaches exist, and risk factors for new infection might change over time. In addition, previous studies that have used a similar approach within a single country had larger sample sizes of recent infections because a larger proportion of HIV infections were classified as recent, such as 7% in Kenya (13) and 17% in Uganda (12). In contrast, only 1.6% of HIV infections in our study were classified as recent infections, likely because incidence rates have declined; the studies in Uganda (12) and Kenya (13) used data collected during 2007, whereas PHIA data used in our study were collected during 2015–2019. Furthermore, differences in RITAs might have contributed to differences in proportions of HIV infections classified as recent; for example, the Uganda study used a different assay, which might have a higher false recency rate than the LAg-avidity EIA (29). Moreover, PHIAs used in our study were conducted with a revised RITA that incorporated viral load (>1,000 copies/mL) and absence of antiretroviral drug metabolites.
Because this study used a pooled analysis, we were limited to factors that were collected consistently across each PHIA, reducing our ability to examine potential relationships between recent infections and other factors, such as mobility, violence, stigma, alcohol use, and sexually transmitted infection symptoms or diagnosis, which were not collected consistently across countries. Other potential risk factors, such as education, had to be regrouped into broad categories that might have limited our analysis of their relationship with recent HIV infection. Furthermore, only countries that completed a PHIA were included in the analysis; therefore, results might not be generalizable to other countries in sub-Saharan Africa. Community-level viremia was calculated at the stratum level, which often represented larger geographic or political areas such as regions or provinces. Finally, a potential for misclassification of potential risk factors in PHIAs existed because of the self-reported nature of risk factors of interest and potential for outcome misclassification by RITA.
Despite those limitations, we successfully identified key factors associated with recent infections among the adult populations of 14 countries that have high HIV burdens. Focusing prevention resources on persons who are at higher risk of acquiring a recent infection should contribute to the continued decline in HIV incidence and, ultimately, to epidemic control. Additional strategies will be needed to monitor recent infections, such as routine surveillance as part of HIV testing services for rapid case and cluster investigations (30–32) or using testing history–based methods to classify recent infections within surveys that do not require the use of a recency assay (33). Those data will provide actionable information for HIV programs regarding new outbreak locations and where prevention resources might be needed most (30).
Dr. Currie is an epidemiologist in the Division of Global HIV and TB, Global Health Center, Centers for Disease Control and Prevention, Atlanta, Georgia, USA. His research interests focus on HIV surveillance in the general population and relationships between behavioral science and communicable diseases.
Acknowledgments
This research was supported by the US President's Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of cooperative agreement nos. U2GGH001226 and U2GGH002108.
B.S.P. receives royalties from the sale of LAg-Avidity EIA kits sold by the manufacturer according to US government policy.
References
- Frank TD, Carter A, Jahagirdar D, Biehl MH, Douwes-Schultz D, Larson SL, et al.; GBD 2017 HIV collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6:e831–59. DOIPubMedGoogle Scholar
- UNAIDS. Fact sheet—World AIDS Day 2022 [cited 2022 Apr 11]. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- Joshi K, Lessler J, Olawore O, Loevinsohn G, Bushey S, Tobian AAR, et al. Declining HIV incidence in sub-Saharan Africa: a systematic review and meta-analysis of empiric data. J Int AIDS Soc. 2021;24:
e25818 . DOIPubMedGoogle Scholar - Ghys PD, Williams BG, Over M, Hallett TB, Godfrey-Faussett P. Epidemiological metrics and benchmarks for a transition in the HIV epidemic. PLoS Med. 2018;15:
e1002678 . DOIPubMedGoogle Scholar - Nabukenya AM, Nambuusi A, Matovu JKB. Risk factors for HIV infection among married couples in Rakai, Uganda: a cross-sectional study. BMC Infect Dis. 2020;20:198. DOIPubMedGoogle Scholar
- Eilami O, Nazari A, Dousti M, Sayehmiri F, Ghasemi M. Investigation of HIV/AIDS prevalence and associated risk factors among female sex workers from 2010 to 2017: a meta-analysis study. HIV AIDS (Auckl). 2019;11:105–17. DOIPubMedGoogle Scholar
- Kabapy AF, Shatat HZ, Abd El-Wahab EW. Attributes of HIV infection over decades (1982-2018): A systematic review and meta-analysis. Transbound Emerg Dis. 2020;67:2372–88. DOIPubMedGoogle Scholar
- Geis S, Maboko L, Saathoff E, Hoffmann O, Geldmacher C, Mmbando D, et al. Risk factors for HIV-1 infection in a longitudinal, prospective cohort of adults from the Mbeya Region, Tanzania. J Acquir Immune Defic Syndr. 2011;56:453–9. DOIPubMedGoogle Scholar
- Olawore O, Tobian AAR, Kagaayi J, Bazaale JM, Nantume B, Kigozi G, et al. Migration and risk of HIV acquisition in Rakai, Uganda: a population-based cohort study. Lancet HIV. 2018;5:e181–9. DOIPubMedGoogle Scholar
- Nsanzimana S, Remera E, Kanters S, Mulindabigwi A, Suthar AB, Uwizihiwe JP, et al. Household survey of HIV incidence in Rwanda: a national observational cohort study. Lancet HIV. 2017;4:e457–64. DOIPubMedGoogle Scholar
- Risher KA, Cori A, Reniers G, Marston M, Calvert C, Crampin A, et al.; ALPHA Network. Age patterns of HIV incidence in eastern and southern Africa: a modelling analysis of observational population-based cohort studies. Lancet HIV. 2021;8:e429–39. DOIPubMedGoogle Scholar
- Mermin J, Musinguzi J, Opio A, Kirungi W, Ekwaru JP, Hladik W, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300:540–9. DOIPubMedGoogle Scholar
- Kim AA, Parekh BS, Umuro M, Galgalo T, Bunnell R, Makokha E, et al.; 2007 KAIS study group. 2007 KAIS study group. Identifying risk factors for recent HIV infection in Kenya using a recent infection testing algorithm: results from a nationally representative population-based survey. PLoS One. 2016;11:
e0155498 . DOIPubMedGoogle Scholar - Voetsch AC, Duong YT, Stupp P, Saito S, McCracken S, Dobbs T, et al. HIV-1 recent infection testing algorithm with antiretroviral drug detection to improve accuracy of incidence estimates. J Acquir Immune Defic Syndr. 2021;87(Suppl 1):S73–80. DOIPubMedGoogle Scholar
- Federal Ministry of Health. Nigeria. Nigeria HIV/AIDS indicator and impact survey (NAIIS) 2018 technical report. October 2019 [cited 2023 Sep 18]. https://ciheb.org/media/SOM/Microsites/CIHEB/documents/NAIIS-Report-2018.pdf
- Sachathep K, Radin E, Hladik W, Hakim A, Saito S, Burnett J, et al. Population-based HIV impact assessments survey methods, response, and quality in Zimbabwe, Malawi, and Zambia. J Acquir Immune Defic Syndr. 2021;87(Suppl 1):S6–16. DOIPubMedGoogle Scholar
- Patel HK, Duong YT, Birhanu S, Dobbs T, Lupoli K, Moore C, et al. A comprehensive approach to assuring quality of laboratory testing in HIV surveys: lessons learned from the population-based HIV impact assessment project. J Acquir Immune Defic Syndr. 2021;87(Suppl 1):S17–27. DOIPubMedGoogle Scholar
- Justman JE, Mugurungi O, El-Sadr WM. HIV population surveys—bringing precision to the global response. N Engl J Med. 2018;378:1859–61. DOIPubMedGoogle Scholar
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al.; HPTN 052 Study Team. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. DOIPubMedGoogle Scholar
- Rodger AJ, Cambiano V, Bruun T, Vernazza P, Collins S, van Lunzen J, et al.; PARTNER Study Group. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316:171–81. DOIPubMedGoogle Scholar
- Brault MA, Spiegelman D, Abdool Karim SS, Vermund SH. Integrating and interpreting findings from the latest treatment as prevention trials. Curr HIV/AIDS Rep. 2020;17:249–58. DOIPubMedGoogle Scholar
- Jia KM, Eilerts H, Edun O, Lam K, Howes A, Thomas ML, et al. Risk scores for predicting HIV incidence among adult heterosexual populations in sub-Saharan Africa: a systematic review and meta-analysis. J Int AIDS Soc. 2022;25:
e25861 . DOIPubMedGoogle Scholar - Ramjee G, Daniels B. Women and HIV in sub-Saharan Africa. AIDS Res Ther. 2013;10:30. DOIPubMedGoogle Scholar
- Joint United Nations Programme on HIV/AIDS. Young people and HIV [cited 2022 May 25]. https://www.unaids.org/sites/default/files/media_asset/young-people-and-hiv_en.pdf
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Women and HIV: a spotlight on adolescent girls and young women. 2019 [cited 2022 May 25]. https://www.unaids.org/sites/default/files/media_asset/2019_women-and-hiv_en.pdf
- Vandormael A, Akullian A, Siedner M, de Oliveira T, Bärnighausen T, Tanser F. Declines in HIV incidence among men and women in a South African population-based cohort. Nat Commun. 2019;10:5482. DOIPubMedGoogle Scholar
- Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;15:
CD003362 . DOIPubMedGoogle Scholar - Hines JZ, Sachathep K, Pals S, Davis SM, Toledo C, Bronson M, et al. HIV incidence by male circumcision status from the population-based HIV impact assessment surveys—eight sub-Saharan African countries, 2015–2017. J Acquir Immune Defic Syndr. 2021;87(Suppl 1):S89–96. DOIPubMedGoogle Scholar
- Duong YT, Qiu M, De AK, Jackson K, Dobbs T, Kim AA, et al. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7:
e33328 . DOIPubMedGoogle Scholar - Kim AA, Behel S, Northbrook S, Parekh BS. Tracking with recency assays to control the epidemic: real-time HIV surveillance and public health response. AIDS. 2019;33:1527–9. DOIPubMedGoogle Scholar
- Suthar AB, Ouk V, Samreth S, Ngauv B, Bain R, Eng B, et al. Programmatic implications of national recent HIV infection surveillance in Cambodia. J Infect Dis. 2023;
jiad082 ; Epub ahead of print. DOIPubMedGoogle Scholar - Telford CT, Tessema Z, Msukwa M, Arons MM, Theu J, Bangara FF, et al. Geospatial transmission hotspots of recent HIV infection—Malawi, October 2019–March 2020. MMWR Morb Mortal Wkly Rep. 2022;71:329–34. DOIPubMedGoogle Scholar
- Gurley SA, Stupp PW, Fellows IE, Parekh BS, Young PW, Shiraishi RW, et al. Estimation of HIV-1 incidence using a testing history-based method; analysis from the population-based HIV impact assessment survey data in 12 African countries. J Acquir Immune Defic Syndr. 2023;92:189–96. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: October 16, 2023
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dustin Currie, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H16-3, Atlanta, GA 30329-4027, USA
Top