Volume 30, Number 1—January 2024
Dispatch
Excess Deaths Associated with Rheumatic Heart Disease, Australia, 2013–2017
Abstract
During 2013–2017, the mortality rate ratio for rheumatic heart disease among Indigenous versus non-Indigenous persons in Australia was 15.9, reflecting health inequity. Using excess mortality methods, we found that deaths associated with rheumatic heart disease among Indigenous Australians were probably substantially undercounted, affecting accuracy of calculations based solely on Australian Bureau of Statistics data.
Rheumatic heart disease (RHD), caused by Streptococcus pyogenes infections, is driven by social determinants of health and disproportionately affects Aboriginal and Torres Strait Islanders in Australia (hereafter Indigenous Australians), causing premature illness and death (1–3). Deaths associated with RHD can be prevented by addressing poor living conditions, treatment delays, racism, and healthcare inaccessibility (2,4–6). Approximately 663 deaths associated with RHD among Indigenous Australians are predicted for 2016–2031 (7). Our previous analysis of persons from 5 jurisdictions in Australia who had RHD, were <65 years of age, and died during 2013–2017 (covering 86% of the Indigenous population) revealed that RHD was the underlying cause of death for only 15.0%; cause of death was recorded as underlying noncardiovascular for 42.7%, and cause of death among Indigenous Australians was missing for 13.7% (2). Thus, the burden of death associated with RHD is potentially underestimated when measured by using RHD-coded death records from the Australian Bureau of Statistics (ABS). Concerns regarding inaccurate or missing cause-of-death data can be reduced by using excess mortality methods, which measure deaths directly and indirectly attributable to RHD (8). Consequently, we used excess mortality methods, independent of ABS RHD-coded records, to estimate RHD-associated deaths for 2013–2017 in Australia.
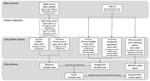
In a cross-sectional study, we used linked administrative health and ABS data to estimate RHD-related deaths (Figure 1). We estimated observed mortality rates by age at death and Indigenous status by using data from End RHD in Australia: Study of Epidemiology (ERASE) (9). We used the generated excess deaths rates to calculate expected RHD-associated deaths and compared them with ABS RHD-coded death counts.
The ERASE cohort has been described (2). In brief, prevalent and new RHD cases were identified from the RHD register, surgical registry, and hospitalization records (1,9,11,12). ERASE included 5 jurisdictions in Australia: Northern Territory, Queensland, South Australia, Western Australia, and New South Wales (Appendix Figure 1). We obtained probabilistically linked data from jurisdiction-specific linkage units; ERASE investigators harmonized variables between jurisdictions and data sources and determined vital status.
To create the RHD study cohort, we selected ERASE cohort members who had RHD, were <65 years of age, and were alive on January 1, 2013 (Figure 1). We used broad age groups (0–24, 25–44, and 45–64 years), which corresponded to those used in previous RHD mortality studies (2,13). We used multiple ERASE data sources to assign Indigenous status, minimizing known underidentification (9). We searched all hospitalization record diagnosis fields for comorbidities (Appendix Table 1).
We calculated observed and background mortality rates (both crude and age-specific per 100,000 person-years). We calculated age-standardized mortality rates by using the direct method, standardized to World Health Organization World Standard Population 5-year age groupings for 2000–2025. For observed mortality rates (Figure 1), RHD diagnoses from January 1, 2013, through December 31, 2017, contributed person-time from whichever time was latest (denominators): first diagnosis date or January 1, 2013. Deaths during 2013–2017 contributed to observed mortality rate numerators. For background mortality rates (Figure 1), we used age group–specific deaths of Indigenous and non-Indigenous Australians (numerators) and residential population denominators from the ABS (13).
We calculated excess mortality rates as the difference between the observed and background mortality rates (within matched age/population stratum; Figure 1). We derived 95% CIs by using nonparametric bootstrap methods, assuming a Poisson distribution (Appendix). We calculated expected RHD-related deaths by applying excess mortality rates to person-years within the RHD study cohort age/population stratum (Appendix Table 2). We calculated observed and excess mortality rate ratios (MRRs) with 95% CIs by comparing Indigenous with non-Indigenous populations with RHD.
Epidemiologic, demographic, and clinical characteristics of this cohort are described (Appendix Table 3). Among the 9,342 persons in the RHD study cohort (65.6% female, 24.6% <25 years of age, 55.6% Indigenous), comorbidities included atrial fibrillation (30.5%), heart failure (26.0%), hypertension (23.7%), diabetes (19.4%), chronic kidney disease (17.4%), and chronic obstructive pulmonary disease (10.6%) (Appendix Table 3). The 726 observed cohort deaths occurred most frequently among persons 45–64 years of age (72.3%) and among those who were female (58.7%) (Appendix Table 3). Among the 325 non-Indigenous persons who died, 36.0% were immigrants from low/middle income countries. Metropolitan residents accounted for 14.0% (n = 56) of deaths among Indigenous and 71.4% (n = 232) among non-Indigenous persons. Detailed causes of death within the study cohort were attributed to mostly noncardiovascular causes; most frequent were cancer, diabetes mellitus, and respiratory diseases (2).
In 2013–2017 in Australia, the background mortality rate was 193.6 deaths/100,000 Indigenous person-years and 72.3 deaths/100,000 non-Indigenous person-years (Table). Background age-specific mortality rates increased with advancing age in both populations but were always 2- to 3-fold higher for the Indigenous than non-Indigenous population (Table, Figure 2)
In the RHD study cohort, 401 Indigenous and 325 non-Indigenous persons died, corresponding to observed mortality rates of 1,451 deaths/100,000 Indigenous person-years and 883 deaths/100,000 non-Indigenous person-years (Table). Age-specific mortality rates among Indigenous persons were highest among those 45–64 years of age (4,568 deaths/100,000 person-years; Figure 2); corresponding MRR was 2.13 (95% CI, 1.81–2.52) for Indigenous versus non-Indigenous persons (Table).
For the RHD study cohort, we estimated excess mortality rates of 1,166 deaths/100,000 Indigenous person-years and 771 deaths/100,000 non-Indigenous person-years, generating an MRR of 1.5 (Table). Excess mortality rates were highest among Indigenous persons 45–64 years of age for whom the peak excess MRR of 2.1 was observed (Table; Figure 2). Excess mortality rates applied to RHD study cohort strata estimated that 319 Indigenous and 272 non-Indigenous deaths were directly or indirectly associated with RHD (Table; Appendix Table 4). By comparison, ABS RHD-coded deaths captured 145 Indigenous deaths, less than half the expected cases (174 fewer than expected), but 300 non-Indigenous deaths, approximately the same as expected (28 more).
Accuracy of our estimates is limited by the quality of the coded information within source datasets and constrained by available data, including nonavailability of migrant population denominator information for rate calculations. The RHD mortality rates that we report also do not capture the profound effects that those deaths had on families, communities, and cultures.
After adjusting for background mortality in Indigenous and non-Indigenous populations, we found that excess deaths were higher among persons with RHD. The excess mortality method applied to the RHD study cohort estimates both direct and indirect RHD-associated deaths and reduces concerns regarding misclassified and missing cause of death arising from use of ABS RHD-coded data only. Our method is particularly useful with the Indigenous population, for whom missing ABS RHD-coded data are an issue. RHD is probably not the only underlying driver of observed excess premature deaths; rather, RHD is a potent marker of the inequities experienced by Indigenous Australians and drives excess deaths indirectly in synergy with other chronic health conditions associated with social determinants. Expected deaths among non-Indigenous persons corresponded closely to ABS RHD-coded records; however, among the Indigenous population, excess deaths were more than twice those recorded in ABS (2). Similar to other chronic illnesses (diabetes and dementia [10,14]), the burden of RHD-associated deaths in Australia is potentially underascertained when based exclusively on ABS RHD-coded records, especially among Indigenous persons, for whom cause-of-death data are missing for >10% and multiple comorbidities, along with underlying RHD, contribute to death (2). Before Australia can achieve its RHD elimination goals, improved quality of Indigenous cause-of-death data and identification of synergistic factors contributing to excess RHD-associated deaths are needed (7).
Ms. Stace is a cardiovascular epidemiologist with a background in biostatistics and a PhD candidate within the Cardiovascular Epidemiology Research Centre, School of Population and Global Health, at the University of Western Australia. Her research interest is using linked administrative data to investigate disease progression and complications associated with acute rheumatic fever and RHD among youth in Australia.
Acknowledgments
We thank ABS for providing the customized aggregated RHD-coded death data, in particular Lauren Moran for her expert review. We also thank the staff of the data linkage units of the state and territory governments (Western Australia, South Australia–Northern Territories, New South Wales, Queensland) for linkage of the ERASE project data. We thank the State and Territory Registries of Births, Deaths and Marriages, the State and Territory Coroners, and the National Coronial Information System and the Victorian Department of Justice for enabling Cause of Death Unit Record File data to be used for this project. Furthermore, we thank the data custodians and data managers for providing inpatient hospital and emergency department data (5 states and territories), RHD registers (5 states and territories), the Australian and New Zealand Society of Cardiac and Thoracic Surgeons Cardiac Surgery Database (single registry covering 5 states and territories), the Royal Melbourne Children’s Hospital Paediatric Cardiac Surgery database (single data source for RHD pediatric patients from South Australia and Northern Territory receiving surgical intervention in Melbourne), and the Northern Territory Department of Health primary healthcare data.
The Human Research Ethics Committees of the Health Departments of participating Australian jurisdictions provided approval for the ERASE project, which is registered on the Australian New Zealand Clinical Trials Registry (ACTRN12620000981921). Aboriginal Ethics Committee approval was sought in jurisdictions where operational and support letters were received from peak bodies of the Aboriginal Community Controlled Health Services.
This work was supported by funding from the National Health and Medical Research Council through project grant no. 1146525 and seed funds from the End Rheumatic Heart Disease Centre for Research Excellence and HeartKids.
I.S. is supported by a National Health and Medical Research Council Postgraduate Scholarship (grant no. 2005398) and an ad hoc postgraduate scholarship from The University of Western Australia. J.K. and L.N. are supported by National Heart Foundation Future Leader Fellowships (nos. 102043, 105038).
References
- Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Stacey I, et al. Contemporary incidence and prevalence of rheumatic fever and rheumatic heart disease in Australia using linked data: the case for policy change. J Am Heart Assoc. 2020;9:
e016851 . DOIPubMedGoogle Scholar - Stacey I, Seth R, Nedkoff L, Hung J, Wade V, Haynes E, et al. Rheumatic heart disease mortality in Indigenous and non-Indigenous Australians between 2013 and 2017. Heart. 2023;109:1025–33. DOIPubMedGoogle Scholar
- Stacey I, Hung J, Cannon J, Seth RJ, Remenyi B, Bond-Smith D, et al. Long-term outcomes following rheumatic heart disease diagnosis in Australia. Eur Heart J Open. 2021;1:oeab035.
- Colquhoun SM, Condon JR, Steer AC, Li SQ, Guthridge S, Carapetis JR. Disparity in mortality from rheumatic heart disease in Indigenous Australians. J Am Heart Assoc. 2015;4:
e001282 . DOIPubMedGoogle Scholar - Coroners Court of Queensland. Inquest into the deaths of Yvette Michelle Wilma Booth, Adele Estelle Sandy, Shakaya George (“RHD Doomadgee Cluster”) 2023 [cited 2023 Jul 1]. https://www.courts.qld.gov.au/courts/coroners-court
- Wade V, Stewart M. Bridging the gap between science and Indigenous cosmologies: Rheumatic Heart Disease Champions4Change. Microbiol Aust. 2022;43:89–92."https://doi.org/10.1071/MA22030" DOIGoogle Scholar
- Wyber R, Noonan K, Halkon C, Enkel S, Cannon J, Haynes E, et al.; END RHD CRE Investigators Collaborators. Ending rheumatic heart disease in Australia: the evidence for a new approach. Med J Aust. 2020;213(Suppl 10):S3–31. DOIPubMedGoogle Scholar
- Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med. 2006;260:103–17. DOIPubMedGoogle Scholar
- Katzenellenbogen JM, Bond-Smith D, Seth RJ, Dempsey K, Cannon J, Nedkoff L, et al.; ERASE Collaboration Study Group. The End Rheumatic Heart Disease in Australia Study of Epidemiology (ERASE) Project: data sources, case ascertainment and cohort profile. Clin Epidemiol. 2019;11:997–1010. DOIPubMedGoogle Scholar
- Gao L, Calloway R, Zhao E, Brayne C, Matthews FE; Medical Research Council Cognitive Function and Ageing Collaboration. Accuracy of death certification of dementia in population-based samples of older people: analysis over time. Age Ageing. 2018;47:589–94. DOIPubMedGoogle Scholar
- Bond-Smith D, Seth R, de Klerk N, Nedkoff L, Anderson M, Hung J, et al. Development and evaluation of a prediction model for ascertaining rheumatic heart disease status in administrative data. Clin Epidemiol. 2020;12:717–30. DOIPubMedGoogle Scholar
- Katzenellenbogen JM, Nedkoff L, Canon J, Kruger D, Pretty F, Carapetis JR, et al. Low positive predictive value of ICD-10 codes in relation to rheumatic heart disease: a challenge for global surveillance. Int Med J. 2019;49:400–3. DOIPubMedGoogle Scholar
- Australian Bureau of Statistics. National, state, and territory population [cited 2020 Dec 9]. https://www.abs.gov.au/statistics/people/population/national-state-and-territory-population
- Whittall DE, Glatthaar C, Knuiman MW, Welborn TA. Deaths from diabetes are under-reported in national mortality statistics. Med J Aust. 1990;152:598–600. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: December 16, 2023
Table of Contents – Volume 30, Number 1—January 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Ingrid Stacey, Cardiovascular Epidemiology Research Centre, School of Population and Global Health, M431 Clifton Street Bldg, Clifton St, Nedlands, Western Australia 6009, Australia
Top