Volume 30, Number 8—August 2024
Research Letter
Persistence of Influenza H5N1 and H1N1 Viruses in Unpasteurized Milk on Milking Unit Surfaces
Abstract
Examining the persistence of highly pathogenic avian influenza A(H5N1) from cattle and human influenza A(H1N1)pdm09 pandemic viruses in unpasteurized milk revealed that both remain infectious on milking equipment materials for several hours. Those findings highlight the risk for H5N1 virus transmission to humans from contaminated surfaces during the milking process.
Highly pathogenic avian influenza A(H5N1) virus was detected in US domestic dairy cattle in late March 2024, after which it spread to herds across multiple states and resulted in at least 3 confirmed human infections (1). Assessment of milk from infected dairy cows indicated that unpasteurized milk contained high levels of infectious influenza virus (2; L.C. Caserta et al., unpub. data, https://doi.org/10.1101/2024.05.22.595317). Exposure of dairy farm workers to contaminated unpasteurized milk during the milking process could lead to increased human H5 virus infections. Such infections could enable H5 viruses to adapt through viral evolution within humans and gain the capability for human-to-human transmission.
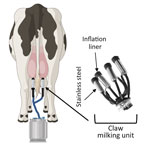
The milking process is primarily automated and uses vacuum units, commonly referred to as clusters or claws, which are attached to the dairy cow teats to collect milk (Figure 1) (3). However, several steps in the milking process require human input, including forestripping, whereby workers manually express the first 3–5 streams of milk from each teat by hand. Forestripping stimulates the teats for optimal milk letdown, improves milk quality by removing bacteria, and provides an opportunity to check for abnormal milk. The forestripping process can result in milk splatter on the floor of the milking parlor and surrounding equipment and production of milk aerosols.
After forestripping, each teat is cleaned and dried by hand before the claw is installed. During milking, a flexible rubber inflation liner housed within the stainless-steel shell of the claw opens to enable the flow of milk and closes to exert pressure on the teat to stop the flow of milk (Figure 1). When the flow of milk decreases to a specific level, the claw automatically releases (3), at which point residual milk in the inflation liner could spray onto dairy workers, equipment, or the surrounding area. Of note, milking often takes place at human eye level; the human workspace is physically lower than the cows, which increases the potential for infectious milk to contact human workers’ mucus membranes. No eye or respiratory protection is currently required for dairy farm workers, but recommendations have been released (4).
Influenza virus persistence in unpasteurized milk on surfaces is unclear, but information on virus persistence is critical to understanding viral exposure risk to dairy workers during the milking process. Therefore, we analyzed the persistence of infectious influenza viruses in unpasteurized milk on surfaces commonly found in milking units, such as rubber inflation liners and stainless steel (Figure 1).
For infectious strains, we used influenza A(H5N1) strain A/dairy cattle/TX/8749001/2024 or a surrogate influenza A(H1N1)pdm09 pandemic influenza virus strain, A/California/07/2009. We diluted virus 1:10 in raw unpasteurized milk and in phosphate-buffered saline (PBS) as a control. As described in prior studies (5–7), we pipetted small droplets of diluted virus in milk or PBS onto either stainless steel or rubber inflation liner coupons inside an environmental chamber. We then collected virus samples immediately (time 0) or after 1, 3, or 5 hours to detect infectious virus by endpoint titration using a 50% tissue culture infectious dose assay (7). To mimic environmental conditions within open-air milking parlors in the Texas panhandle during March–April 2024, when the virus was detected in dairy herds, we conducted persistence studies using 70% relative humidity.
We observed that the H5N1 cattle virus remained infectious in unpasteurized milk on stainless steel and rubber inflation lining after 1 hour, whereas infectious virus in PBS fell to below the limit of detection after 1 hour (Figure 2, panel A). That finding indicates that unpasteurized milk containing H5N1 virus remains infectious on materials within the milking unit. To assess whether a less pathogenic influenza virus could be used as a surrogate to study viral persistence on milking unit materials, we compared viral decay between H5N1 and H1N1 in raw milk over 1 hour on rubber inflation liner and stainless-steel surfaces (Figure 2, panel B). The 2 viruses had similar decay rates on both surfaces, suggesting that H1N1 can be used as a surrogate for H5N1 cattle virus in studies of viral persistence in raw milk. Further experiments examining H1N1 infectiousness over longer periods revealed viral persistence in unpasteurized milk on rubber inflation liner for at least 3 hours and on stainless steel for at least 1 hour (Figure 2, panel C). Those results indicate that influenza virus is stable in unpasteurized milk and that influenza A virus deposited on milking equipment could remain infectious for >3 hours.
Taken together, our data provide compelling evidence that dairy farm workers are at risk for infection with H5N1 virus from surfaces contaminated during the milking process. To reduce H5N1 virus spillover from dairy cows to humans, farms should implement use of personal protective equipment, such as face shields, masks, and eye protection, for workers during milking. In addition, contaminated rubber inflation liners could be responsible for the cattle-to-cattle spread observed on dairy farms. Sanitizing the liners after milking each cow could reduce influenza virus spread between animals on farms and help curb the current outbreak.
Dr. Le Sage is a research assistant professor at the University of Pittsburgh Center for Vaccine Research, Pittsburgh, Pennsylvania, USA. Her research interests include elucidating the requirements for influenza virus transmission and assessing the pandemic potential of emerging influenza viruses.
Acknowledgments
We thank the Lakdawala lab members, Centers of Excellence for Influenza Research and Response (CEIRR) risk assessment pipeline meeting attendees, Rachel Duron, and Linsey Marr for useful feedback.
This project was funded in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no. 75N93021C00015 and a National Institutes of Health award (no. UC7AI180311) from the National Institute of Allergy and Infectious Diseases supporting the operations of the University of Pittsburgh Regional Biocontainment Laboratory in the Center for Vaccine Research. H5N1 studies were performed in accordance with select agent permit no. 20230320-074008 at the University of Pittsburgh.
This article was preprinted at https://www.medrxiv.org/content/10.1101/2024.05.22.24307745v1.
References
- Centers for Disease Control and Prevention. H5N1 bird flu: current situation summary [cited 2024 Jun 13]. https://www.cdc.gov/flu/avianflu/avian-flu-summary.htm
- Burrough ER, Magstadt DR, Petersen B, Timmermans SJ, Gauger PC, Zhang J, et al. Highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus infection in domestic dairy cattle and cats, United States, 2024. Emerg Infect Dis. 2024;30:1335–43. DOIPubMedGoogle Scholar
- Odorčić M, Rasmussen MD, Paulrud CO, Bruckmaier RM. Review: Milking machine settings, teat condition and milking efficiency in dairy cows. Animal. 2019;13(S1):s94–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Avian influenza (bird flu): reducing risk for people working with or exposed to animals [cited 2024 Jun 20]. https://www.cdc.gov/bird-flu/prevention/worker-protection-ppe.html
- Qian Z, Morris DH, Avery A, Kormuth KA, Le Sage V, Myerburg MM, et al. Variability in donor lung culture and relative humidity impact the stability of 2009 pandemic H1N1 influenza virus on nonporous surfaces. Appl Environ Microbiol. 2023;89:
e0063323 . DOIPubMedGoogle Scholar - Kormuth KA, Lin K, Qian Z, Myerburg MM, Marr LC, Lakdawala SS. Environmental persistence of influenza viruses is dependent upon virus type and host origin. MSphere. 2019;4:e00552–19. DOIPubMedGoogle Scholar
- Kormuth KA, Lin K, Prussin AJ II, Vejerano EP, Tiwari AJ, Cox SS, et al. Influenza virus infectivity is retained in aerosols and droplets independent of relative humidity. J Infect Dis. 2018;218:739–47. DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: June 24, 2024
1These first authors contributed equally to this article.
Table of Contents – Volume 30, Number 8—August 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Seema Lakdawala, Emory University School of Medicine, 1510 Clifton Rd, Rm 3121, Rollins Research Center, Atlanta, GA 30322, USA
Top