Volume 6, Number 4—August 2000
Dispatch
Borrelia burgdorferi and the Causative Agent of Human Granulocytic Ehrlichiosis in Deer Ticks, Delaware
Abstract
During the 1998 hunting season in Delaware, 1,480 ticks were collected from 252 white-tailed deer; 98% were Ixodes scapularis, a significant increase from the 85% reported in 1988. Ticks were tested for Borrelia burgdorferi and the causative agent of human granulocytic ehrlichiosis. Infection rates remained stable in New Castle and Kent Counties, but increased from < 1% to 8% in Sussex County.
Delaware has one of the highest incidence rates of Lyme disease in the United States, with an average annual rate over the past 10 years of 14.43 cases/100,000 (1). Ixodes scapularis, the primary vector of Borrelia burgdorferi, the causative agent of Lyme disease, has also been implicated in transmission of the causative agent of human granulocytic ehrlichiosis (HGE) (2). From 1986 to 1997, 449 cases of HGE were reported in the United States (3), none of them in Delaware, where HGE is not a reportable illness.
To determine if there has been an increase in I. scapularis or B. burgdorferi infection rate and if the causative agent of HGE is present in Delaware, we tested ticks by polymerase chain reaction (PCR) for both B. burgdorferi and the causative agent of HGE (4,5). The only available data on B. burgdorferi infection rates and tick density in Delaware are from a 1988 study of ticks parasitizing hunter-harvested white-tailed deer, Odocoileus virginianus (6).
During the 1998 hunting season, hunters brought deer to six check stations operated in three counties by the Delaware Division of Natural Resources. Deer were inspected for ticks during November 13-21, 1998. Information collected for each deer included the county of origin, sex, and approximate age and weight. As in the 1988 study, one side of the head, neck, and shoulder of each deer was combed for ticks. All ticks were placed in 70% ethanol and held for identification. The percentage of I. scapularis among the ticks collected in each county and statewide was compared with the percentage reported in the 1988 study (Z-test for comparisons among proportions) (7).
Because heme can interfere with PCR accuracy (8), engorged ticks were excluded from testing. Fifty I. scapularis ticks were randomly selected from each of the three Delaware counties from the remaining males and unengorged females.
Bacterial DNA was recovered from the ticks by methods modified from those described by Persing et al. (9) were used to recover from ticks. Briefly, ticks were placed in a sterile microfuge tube along with 20 µl of sterile 0.5-mm glass beads treated with 1% bovine albumin. Sterile 0.1M Tris buffer (25 µl, pH 8.3) was added, and the ticks were crushed into the beads for one min to release the midgut contents. Samples were boiled for 5 min, immediately placed in ice water for 2 min, and held at 4°C until used for PCR.
For B. burgdorferi, oligonucleotide primers OSP-A1 and OSP-A2 (5) were obtained from Only DNA (Midland, TX). PCR was performed in a Hybaid thermal cycler by using 0.5 M of each primer, 5 µl of tick extract (template), and 25 µl REDTAQ PCR mixture (Sigma). Components were denatured at 94°C for 2 min and then subjected to 30 cycles of denaturing (94°C for 30 sec), annealing (54°C for 30 sec), and extension (72°C for 2 min). Samples were analyzed by electrophoresis on 0.8% agarose, stained with ethidium bromide, and viewed on a UV transilluminator. Samples were considered positive if the expected 158-bp fragment was seen.
For the HGE agent, we used a nested PCR as described by Massung et al. (10). Primers ge3a and ge10r were obtained from Only DNA (Midland, TX). The first round of PCR was carried out in a Hybaid thermal cycler with 0.5 M of each primer, 5 µl of tick extract (template), and 25 µl of REDTAQ PCR mixture (Sigma). Components were denatured at 95°C for 2 min and then subjected to 40 cycles of denaturing (94°C for 30 sec), annealing (55°C for 30 sec), and extension (72°C for 2 min). Samples were analyzed by electrophoresis on 0.8% agarose, stained with ethidium bromide, and viewed on a UV transilluminator. Samples were considered positive if the expected 932-bp fragment was seen.
Positive specimens were analyzed in a second round of PCR with primers ge9f and ge2 (10) from Only DNA (Midland, TX) and 5 µl of the positive primary PCR solution. Amplification and analysis conditions were identical to those of the first round, except 30 cycles were used. Samples were considered positive if the expected 546-bp product was seen in the second round of PCR. Quality control measures included positive controls from infected I. scapularis nymphs maintained in colonies at Yale University, as well as from field-collected adults. Negative samples were reagent blanks with buffer instead of tick extract.
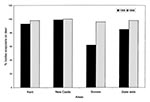
During November 13-21, 1998, 252 deer were examined, and 1,480 ticks were collected (Table). The percentage of I. scapularis collected in New Castle County remained stable from 1988 (99%) to 1998 (98%) (Figure 1). In Kent County, Sussex County, and statewide, the proportion of I. scapularis increased significantly, from 93% in 1988 to 100% in 1998 (z = 5.0, µ = 0.05). In Sussex County in 1988, 62% of the ticks collected were I. scapularis; by 1998 the proportion was 96% (z = 17.0, µ = 0.05). Statewide, the proportion of I. scapularis also rose significantly, from 85% in 1988 to 98% in 1998 (z = 13.3, µ = 0.05).
Of the 50 ticks tested from New Castle County, six were positive for B. burgdorferi and two for the HGE agent. In both Kent and Sussex Counties, 4 of the 50 ticks tested positive for B. burgdorferi, while none tested positive for the HGE agent. None were positive for both organisms.
This study confirms the presence of the causative agent of HGE in New Castle County, Delaware. Although the infection rate in ticks is low (4%), physicians should be aware of the risk for this disease. In Alabama, where an infection rate of 3% was reported for B. burgdorferi in I. scapularis (11), 54 cases of Lyme disease were reported by 1996 (12). Most confirmed cases of HGE occur in states that, like Delaware, have high incidence rates of Lyme disease (13). Although neither of the HGE-positive ticks from Delaware tested positive for B. burgdorferi, such simultaneous infection has been reported elsewhere in ticks (3,14,15), as well as humans (16).
In Delaware, Lyme disease was reported more frequently in 1998 than in 1988 (1). The largest increase in the B. burgdorferi infection rate corresponds with the greatest increase in I. scapularis. Sussex County had the largest increase in the proportion of I. scapularis among ticks found on deer. In 1988, 62% of the ticks parasitizing deer were I. scapularis; this proportion increased to 96% by 1998 (Figure 1). Sussex County also had the largest increase in B. burgdorferi infection rates (Figure 2). Our data indicate that the high Lyme disease incidence may be attributed to an increase in the B. burgdorferi infection rate in I. scapularis in some areas. Previous studies have demonstrated a correlation between the number of cases of Lyme disease and the numbers of I. scapularis (17,18).
To test ticks concurrently for both B. burgdorferi and the causative agent of HGE, we used PCR, while the 1988 study used immunofluorescent assay (2). Comparing infection rates obtained by different procedures may be problematic; for example, the elevated infection rate of B. burgdorferi in Sussex County could result from the greater sensitivity of PCR (15). However, we saw a large increase in infection rate only in Sussex County, where there is a corresponding increase in the proportion of I. scapularis. Our results show that at least in Sussex County I. scapularis is more common, the infection rate for B. burgdorferi is higher, and thus the risk for Lyme disease in Sussex County is higher now than it was 10 years ago. To allow comparison of infection rates between studies, we examined ticks parasitizing deer, even though a better assessment of human risk would have been to examine infection rates in questing ticks.
The distribution of Lyme disease and HGE in Delaware remains poorly defined. Examining ticks collected from hunter-harvested animals provides only a rough indication of where the ticks and bacteria occur. Deer can travel great distances; hunters may not reveal the exact location of the hunt; and infection rates can vary within the same region (15). Therefore, relying on ticks collected from deer does not provide sufficient information about specific locations where risk for exposure to Lyme or HGE may be elevated.
Acknowledgment
The authors thank Ken Reynolds of the Delaware Division of Fish and Wildlife for access to the state-run deer check stations; Timothy Clark, Benjamin Groh, Nate Reynolds, Adam Ritzenthaler, Maria Van Meter, and Elizabeth Willey for assisting with tick collections; Durland Fish of Yale University for providing the ticks used for positive controls; and William Kroen, Bruce Allison, and two anonymous reviewers for critical reading of the manuscript.
References
- Centers For Disease Control and Prevention. Lyme disease: epidemiology. 1999. [cited 1999 Dec 1] Available from URL: http://www.cdc.gov/ncidod/dvbid/lymeepi.htm.
- Pancholi P, Kolbert CP, Mitchell PD, Reed KD, Dumler JS, Bakken JS, Ixodes dammini as a potential vector of human granulocytic ehrlichiosis. J Infect Dis. 1995;172:1007–12.PubMedGoogle Scholar
- McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–42. DOIPubMedGoogle Scholar
- Persing DH, Telford SR, Spielman A, Barthold SW. Detection of Borrelia burgdorferi infection in Ixodes dammini ticks with the polymerase chain reaction. J Clin Microbiol. 1990;28:566.PubMedGoogle Scholar
- Magnarelli LA, Stafford KC, Mather TN, Yeh MT, Horn KD, Dumler JS, Hemocytic Rickettsia-like organisms in ticks: serologic reactivity with antisera to ehrlichiae and detection of DNA of agent of human granulocytic ehrlichiosis by PCR. J Clin Microbiol. 1995;33:2710–4.PubMedGoogle Scholar
- Wolfe D, Fries C, Reynolds K, Hathcock L. The epidemiology of Lyme disease in Delaware 1989-1992. Del Med J. 1994;66:603–13.PubMedGoogle Scholar
- Walpole RE, Myers RH. Probability and statistics for engineers and scientists. 2nd edition. New York: Macmillan Publishing Co., Inc. 1978.
- Schwartz IS, Varde S, Nadelman RB, Wormser GP, Fish D. Inhibition of efficient polymerase chain reaction amplification of Borrelia burgdorferi DNA in blood fed ticks. Am J Trop Med Hyg. 1997;56:339–42.PubMedGoogle Scholar
- Persing DH, Telford SR, Rys PN, Dodge DE, White TJ, Malawista SE, Detection of Borrelia burgdorferi DNA in museum specimens of Ixodes dammini ticks. Science. 1990;249:1420. DOIPubMedGoogle Scholar
- Massung RF, Slater K, Owens JH, Nicholson WI, Mather TN, Solberg VB, Nested PCR assay for detection of granulocytic Ehrlichia. J Clin Microbiol. 1998;36:1090.PubMedGoogle Scholar
- Luckhart S, Mullen GR, Wright JC. Etiological agent of Lyme disease, Borrelia burgdorferi, detected in ticks (Acari:Ixodidae) collected at a focus in Alabama. J Med Entomol. 1991;28:652–7.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Lyme disease--United States, 1996. MMWR Morb Mortal Wkly Rep. 1997;46:531–5.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Statewide surveillance for ehrlichiosis--Connecticut and New York, 1994-1997. MMWR Morb Mortal Wkly Rep. 1998;47:476–80.PubMedGoogle Scholar
- Varde S, Beckley J, Schwartz I. Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey county. Emerg Infect Dis. 1998;4:97–9. DOIPubMedGoogle Scholar
- Daniels TJ, Boccia TM, Varde S, Marcus J, Le J, Bucher DJ, Geographic risk for Lyme disease and human granulocytic ehrlichiosis in southern New York state. Appl Environ Microbiol. 1998;64:4663–9.PubMedGoogle Scholar
- Nadelman RB, Horowitz HW, Hsieh TC, Wu JM, Aguero-Rosenfeld ME, Schwartz I, . Simultaneous human granulocytic ehrlichiosis and Lyme borreliosis. N Engl J Med. 1997;337:27–30. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Lyme disease--United States, 1987-1988. MMWR Morb Mortal Wkly Rep. 1989;38:668–72.PubMedGoogle Scholar
- Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D, Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with Erythema migrans. Am J Epidemiol. 1999;149:771–6.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 6, Number 4—August 2000
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for Correspondence: Kathleen L. Curran, Department of Biology, Wesley College, 120 North State Street, Dover, DE 19901; fax: 302-736-2301
Top