Volume 10, Number 5—May 2004
Dispatch
Domestic Poultry and SARS Coronavirus, Southern China
Abstract
SARS coronavirus injected intratracheally into chickens, turkeys, geese, ducks, and quail, or into the allantoic sac of their embryonating eggs, failed to cause disease or replicate. This finding suggests that domestic poultry were unlikely to have been the reservoir, or associated with dissemination, of SARS coronavirus in the animal markets of southern China.
An outbreak of severe acute respiratory syndrome (SARS) occurred in Guangdong Province, People’s Republic of China, in November 2002 and spread to patients in 30 countries in Africa, Asia, Australia, Europe, and North and South America (1,2). As of July 11, 2003, SARS had been diagnosed in 8,437 patients; 813 died (1). A novel coronavirus was isolated in tissue culture or detected by reverse transcription–polymerase chain reaction (RT-PCR) from multiple respiratory specimens in many patients with SARS (2–4). The SARS-coronavirus (SARS-CoV) is proposed to be the cause of this syndrome on the basis of its association with human clinical cases (3,4) and reproduction of pulmonary lesions in experimentally challenged cynomolgus macaque monkeys (Macaca fascicularis) (5). Furthermore, some of the first persons identified with SARS-CoV infections were vendors in animal markets of southern China, which suggests a possible animal source (6). SARS-CoV has been detected by real-time RT-PCR or isolated from two wild mammalian species, Himalayan palm civet (Paguma larvata) and raccoon dog (Nytereutes procyonoides), in a market in southern China (7), but other studies in southern China involving six provinces and Beijing, as well as sampling of 54 wild and 11 domestic animal species, did not find SARS-CoV (8). The original source of this virus remains unknown (3). The susceptibility of different animal species within the animal meat markets is unknown.
Coronaviruses have been identified in numerous mammalian and avian hosts. Most widely studied and of common occurrence are coronaviruses reported in chickens (Infectious bronchitis virus), turkeys (turkey enteric coronaviruses), cats (feline infectious peritonitis virus and feline enteric coronavirus), dogs (canine enteric coronaviruses), swine (Porcine hemagglutinating encephalomyelitis virus, porcine transmissible gastroenteritis virus, and porcine respiratory coronavirus), cattle (bovine enteric and respiratory coronaviruses), mice (Murine hepatitis virus), rats (sialodacyradenitis virus), rabbits (rabbit coronavirus), and humans (respiratory and enteric coronaviruses) (9). However, on the basis of sequence data, SARS-CoV is sufficiently different from these known group 1, 2, and 3 animal and human coronaviruses to be classified as a new group, group 4 coronaviruses (10). Most likely SARS-CoV originated from an unknown animal reservoir, not from a benign coronavirus in the human population (10,11).
Domesticated poultry species are major commodities traded in the animal markets of southern China. Poultry have been shown to be reservoirs for H5N1 and H9N2 avian influenza viruses that have crossed over and caused infections in humans from 1997 to 2003, some with fatal outcomes (12–14). Therefore, poultry should be examined as potential hosts for infection and amplification of SARS-CoV to determine any potential role they may have played during the emergence of human infections in southern China.
Groups of nine 3-week-old domestic geese (Anser anser domesticus), 3-week-old domestic Pekin ducks (Anas platyrhyncos), 4-week-old chickens (Gallus gallus domesticus), 3-week-old turkeys (Meleagris gallopavo), and 5-week-old Japanese quail (Coturnix coturnix japonicus) were each injected intratracheally with 106.2 mean tissue culture infective doses (TCID50) of Vero E6 propagated Urbani SARS-CoV per bird in a volume of 0.1 mL. The inoculum was the third passage in Vero E6 cells from the original throat swab specimen of the patient. The chickens were specific pathogen–free from an inhouse flock. The other four species were conventional birds obtained at 1 day (geese, turkeys, and ducks) or 5 weeks of age (quail) from commercial hatcheries and raised on site. Oropharyngeal and cloacal swabs were obtained on days 0, 1, 2, 3, 4 and 10 after injection from five birds per group for virus detection by real-time RT-PCR and virus isolation on Vero E6 cells. RNA for RRT-PCR was extracted with the Trizol LS reagent (Invitrogen, Carlsbad, CA) in accordance with the manufacturer’s instructions. Two hydrolysis probe type real-time RT-PCR assays, both targeting the ORF 1b gene, were optimized and run on a Smart Cycler (Cepheid, Sunnyvale, CA) with the superscript platinum taq one-step RT-PCR kit (Invitrogen, Carlsbad, CA). Real-time RT-PCR tests included negative (noninfected tissue culture media, infectious bronchitis coronavirus, and turkey enteric coronaviruses) and positive (Vero E6 propagated SARS-CoV) controls. Two injected birds of each species were euthanized. After necropsy, their tissues were collected for histopathologic examination (all tissue types) and virus detection (plasma, trachea, lung, spleen, kidney, and heart) on days 2 and 4 after injection, and at termination on day 10 after injection. For determination of infection, serum was collected on days 0 and 10 after injection from all birds and tested by indirect enzyme-linked immunosorbent assay for anti-SARS-CoV antibodies. Antigen used to coat plates was tissue culture propagated Urbani strain of SARS-CoV inactivated by γ irradiation (3). Secondary “anti-bird” antibody (Bethyl Laboratories, Montgomery, TX) for testing quail and goose serum or plasma, and secondary anti-duck, anti-chicken, and anti-turkey antibodies (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) for testing duck, chicken, and turkey serum and plasma, respectively, were used. Two birds of each species received uninoculated tissue culture fluid and served as the sham-inoculated groups for real-time RT-PCR, standard RT-PCR, virus isolation, and histopathologic and serologic assays.
To determine if SARS-CoV could grow in avian embryos, 9-day-old chicken eggs and 13-day-old turkey embryonating eggs were inoculated by allantoic sac route and 17-day embryonating turkey eggs were inoculated by yolk sac route; all were tested by virus isolation and real-time RT-PCR for SARS-CoV. All laboratory procedures and animal studies were conducted in biosafety level 3 agriculture (BSL-3AG) (15) facility with HEPA respiratory protection and barrier clothing procedures for personnel. General care was provided in accordance with the Institutional Animal Care and Use Committee.
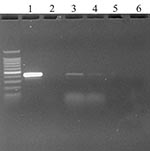
To establish the comparative sensitivity of virus isolation and real-time RT-PCR tests, serial dilutions of SARS-CoV propagated in E6 Vero cell culture were tested for virus reisolation in E6 Vero cells and detection of replicase ORF 1b gene by real-time RT-PCR (16). Virus isolation was slightly more sensitive, detecting virus in two of three replicates at the 10-7 dilution; the real-time RT-PCR test detected SARS-CoV in three of three replicates at 10-5 to 10-6 dilution, depending on primer sets. The real-time RT-PCR assay detected virus in oropharyngeal swab specimens from two chickens on day 1 PI (Figure). Real-time RT-PCR results were confirmed by standard RT-PCR targeting the same gene (primers: SARS clone1b For 5′- TgACAgAgCCATgCCT-3′, SARS clone1b Rev 5’CAACggCATCATCAgA-3′) and sequencing of the amplified product. No infectious virus was isolated from any of the birds at any time from oropharyngeal or cloacal swab specimens, plasma, or tissues. Histologic examination did not identify any specific lesions. No anti–SARS-CoV–specific antibodies were detected in birds at 0 or 10 days after injection. Levels of SARS-CoV were detected corresponding to the inoculated titers in chicken and turkey embryonating eggs by real-time RT-PCR, but not by virus isolation.
These findings suggest that poultry were unlikely to have been infected during the recent SARS-CoV outbreak and were unlikely to have played any role as amplifiers in the animal markets of southern China. The low level of virus detected by real-time RT-PCR from the chickens and the failure to isolate virus from embryonating chicken and turkey eggs suggest that the detected virus was residual inoculum or nonviable virus and that substantial virus replication in the poultry was unlikely. In addition, this SARS-CoV was of low tissue culture passage, i.e., third passage in Vero E6 cell, which minimized the potential for increased cell culture adaptation and concomitant decrease in vivo replication. Using the original or second tissue culture passage would unlikely have resulted in substantial replication in poultry. However, the virus used in these experiments, the Urbani SARS-CoV, had a 29-nt deletion in the genome. Whether the GZ01 human virus or those from civet cats and raccoon dog containing the extra 29 nt would infect and amplify in poultry would be of interest for future research.
Dr. Swayne is a veterinary pathologist and the director of the Southeast Poultry Research Laboratory of the Agricultural Research Service, U.S. Department of Agriculture. His research focuses on pathobiology and control of exotic and emerging viral diseases of poultry and other birds, principally highly pathogenic avian influenza, avian metapneumovirus, and West Nile virus.
Acknowledgments
We thank Suzanne DeBlois and Scott Lee for excellent technical assistance.
Funding for this study was provided by the U.S. Department of Agriculture, Agricultural Research Service CRIS project #6612-32000-039.
References
- World Health Organization. Cumulative number of reported probable cases of SARS. [accessed July 6, 2003]. Available from: http://www.who.int/csr/sars/country/2003_07_11/en/
- Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. DOIPubMedGoogle Scholar
- Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. DOIPubMedGoogle Scholar
- Drosten C, Gunther S, Preiser W, van der Werf S, Brodt HR, Becker S, Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. DOIPubMedGoogle Scholar
- Fouchier RA, Kuiken T, Schutten M, van Amerongen G, van Doornum GJ, van den Hoogen BG, Aetiology: Koch’ postulates fulfilled for SARS virus. Nature. 2003;423:240. DOIPubMedGoogle Scholar
- Field H. The role of animals in transmission of SARS. [accessed June 20, 2003]. Available from: http://www.who.int/csr/sars/conference/june_2003/materials/presentations/en/
- Guan Y, Zheng BJ, He YQ, Liu XL, Zhuang ZX, Cheung CL, Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302:276–8. DOIPubMedGoogle Scholar
- Normile D, Enserink M. SARS in China. Tracking the roots of a killer. Science. 2003;301:297–9. DOIPubMedGoogle Scholar
- Holmes KV. Coronaviruses. In: Granoff A, Webster RG, editors. Encyclopedia of virology. San Diego: Academic Press; 1999. p. 291–8.
- Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. DOIPubMedGoogle Scholar
- Rota PA, Oberste MS, Monroe SS, Nix WA, Campagnoli R, Icenogle JP, Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Influenza A (H9N2) infections in Hong Kong. [accessed June 20, 2003]. Available from: http://www.cdc.gov/ncidod/diseases/flu/H9N2Info.htm
- World Health Organization. WHO news: Avian influenza virus reappears in Hong Kong Special Administrative Region. Bull World Health Organ. 2003;81:232.
- Centers for Disease Control and Prevention. Isolation of avian influenza A (H5N1) from humans—Hong Kong, May–December, 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1204–7.PubMedGoogle Scholar
- Barbeito MS, Abraham G, Best M, Cairns P, Langevin P, Sterritt WG, Recommended biocontainment features for research and diagnostic facilities where animal pathogens are used. Rev Sci Tech Off Int Epiz. 1995;14:873–87.PubMedGoogle Scholar
- Emery SL, Erdman DD, Meyer RF, Bowen MD, Tong S, Cook B, Real-time reverse transcription-polymerase chain reaction assay for the SARS-associated coronavirus. Emerg Infect Dis. 2004;10:311–6.PubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 10, Number 5—May 2004
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
David E. Swayne, USDA, ARS, SEPRL, 934 College Station Road, Athens, GA 30605, USA; fax: 706-546-3161
Top