Volume 13, Number 10—October 2007
Dispatch
Borrelia burgdorferi Infection and Cutaneous Lyme Disease, Mexico
Abstract
Four patients who had received tick bites while visiting forests in Mexico had skin lesions that met the case definition of erythema migrans, or borrelial lymphocytoma. Clinical diagnosis was supported with histologic, serologic, and molecular tests. This study suggests the Borrelia burgdorferi infection is in Mexico.
Lyme disease is the most frequently reported vectorborne infectious disease in the United States and Europe (1,2). Studies have suggested that Borrelia burgdorferi infection might be endemic to Mexico (3,4). We searched for histologic, immunologic, and molecular evidence of B. burgdorferi infection in patients with cutaneous manifestations suggestive of Lyme disease in Mexico.
From June 1999 to October 2000, 4 patients in Mexico City had clinical manifestations suggestive of Lyme disease (5,6). Two (36 and 54 years of age) had erythema migrans lesions, and 2 (9 and 34 years of age) had borrelial lymphocytoma lesions. Two reported having been bitten by a hard tick; the other 2, by a nonflying insect. Bites occurred while camping in forests: 3 near Mexico City (National Park La Marquesa) and 1 in Quintana Roo, a southern state in Mexico. All patients lived in Mexico City and had never traveled outside Mexico.
Two patients were treated for acute skin lesions (consistent with erythema migrans), malaise, and arthralgia. The skin lesion was an erythematous macula with regular, reddish edges and a pink center. One patient had a 5-cm lesion on the left forearm; the other had a 6-cm lesion on the left thigh. For the 2 other patients, a nodular erythematous cutaneous lesion (consistent with lymphocytoma), 0.5–2 cm in diameter with regular edges, developed 2 months after the bite. One patient’s lesion was on the earlobe; the other’s, on the left cheek.
Serum from each patient was tested for immunoglobulin M (IgM) and IgG against B. burgdorferi sensu lato by using a commercial ELISA (cutoff optical density 0.200 and indeterminate 0.200–0.400) (Enzygnost Borreliosis, Dade Behring, Marburg, Germany) (7). A Treponema pallidum ELISA (Abbott Murex, Wiesbaden, Germany) was performed to rule out cross-reaction with T. pallidum infection. Serum samples positive for B. burgdorferi by ELISA were further tested by Western blot (WB) by using the Marxblot assay (MarDx Diagnostics, Carlsbad, CA, USA) and Centers for Disease Control and Prevention (CDC) criteria (5).
Serum samples from the 2 lymphocytoma patients were positive for B. burgdorferi by ELISA and WB (Figure 1, panel A; Table). For the 2 erythema migrans patients, serum samples taken 2 weeks after the tick bite were negative for B. burgdorferi IgM and IgG; but 2 months later, 1 patient became seropositive, confirmed by WB (Figure 1, panel B; Table).
Histologic examination of skin biopsy specimens from each erythema migrans lesion showed a mononuclear cell infiltrate in the superficial and deep dermis; infiltrate included lymphocytes and plasma cells around the perivascular zones. Biopsy samples of lymphocytoma lesions showed dense nodular lymphocytic infiltrates in the reticular dermis with well-delineated lymphoid follicles, no atypical mitosis, B-lymphocytes (anti-CD20, DAKO, Carpentería, CA, USA) in the germinal center (Figure 2, panel A), T-lymphocytes (anti-CD45 RO+) in the follicular zone (Figure 2, panel B), and no CD3+ cells.
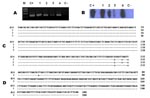
DNA was extracted from the biopsy samples (Repli-g Mini Kit, QIAGEN, Valencia, CA, USA) and used for PCR amplification of a fragment of fla gene specific for B. burgdorferi sensu lato species as well as for a fragment of ospA gene, as described (8–10). DNA from a skin biopsy of a patient with systemic lupus erythematous was used as negative control, and DNA (10 pg/µL) from B. burgdorferi sensu stricto B31 served as positive control. All procedures from DNA extraction to amplification were performed twice for each sample. Amplified products were further tested by Southern blot (SB) hybridization with probes specific for B. burgdorferi sensu stricto, B. garinii, and B. afzelii, as described (9). DNA from the 4 biopsy samples was positive for B. burgdorferi sensu lato fla gene by PCR and confirmed as B. burgdorferi sensu stricto by SB (Appendix Figure; Table). All DNA biopsy samples were negative by SB with the probes specific for B. garinii and B. afzelii. We were able to amplify the OspA gene for only 1 case of erythema migrans, by using PCR and SB tests (data not shown).
The PCR products of the fla gene from 3 patients and of the ospA gene from 1 patient were sequenced by using a commercial kit (GenomeLab DTCS-Quick Start Kit, Beckman Coulter, Inc., Fullerton, CA, USA) with the sequencer from Beckman Coulter, Inc., according to manufacturer’s instructions. We used the DNAMAN program (Lynnon Corporation, Vaudreuil-Dorion, Quebec, Canada) to align the sequences with the reported sequences of the B. burgdorferi sensu stricto B31 strain (Appendix Figure). For the 2 erythema migrans cases, we found 3 base substitutions (Appendix Figure, panel C), 1 of which was not conserved, leading to a change in amino acid (G for R in the 75 aa); these 2 sequences had 99% homology with the sequence of B. burgdorferi fla gene of isolate B31 (BLAST program) (11). For the lymphocytoma case, we found 2 base substitutions, the same as those of the erythema migrans cases, including the nonconserved base substitution (Appendix Figure, panel C).
Regarding the ospA gene in the erythema migrans case, the sequence showed 1 base substitution that was not conserved, leading to a change in the amino acid 5 (L for I). The sequence of this case had 99% homology with the plasmid Ip54 gene of B31strain sequence (11) (Appendix Figure, panel D).
The 3 adult patients received doxycycline 200 mg/day for 3 weeks; the child received amoxicillin 50 mg/kg a day for 3 weeks. For all patients, lesions were gone at the end of the treatment and had not recurred 3 years later.
Erythema migrans is the diagnostic marker for Lyme disease associated with B. burgdorferi infection (5,6). Histologic data from our 2 erythema migrans cases agreed with data reported for other erythema migrans cases (5). Moreover, the 2 erythema migrans cases were positive for B. burgdorferi sensu stricto fla gene and 1 for ospA gene; the 3 cases had a high degree of homology to the sequences of strain B31. In addition, 1 case met CDC criteria for seropositivity to B. burgdorferi infection (5).
Borrelial lymphocytoma is a rare clinical entity reported mostly in Europe (12–14) and sporadically in the United States (15). In this study, histologic and immunohistochemical data from the 2 lymphocytoma cases agreed with data from previous cases. These results were not specific enough to be considered diagnostic; however, germinal centers are present in 80% of borrelial lymphocytoma cases (12). Serum samples from 2 patients were positive by WB, which fulfills CDC criteria (5). In 1 case, fla gene was amplified and sequenced, showing high homology with the fla gene from B. burgdorferi sensu stricto strain B31 (11). Few reports describe genotyping of B. burgdorferi species in borrelial lymphocytoma. In Slovenia, B. afzelii and B. bissettii were identified (13); in Germany, B. garinii was identified (14). In our lymphocytoma patients, we identified B. burgdorferi sensu stricto. That the 2 borrelial lymphocytoma cases occurred in patients who had visited the same national park suggests that B. burgdorferi is endemic to that area.
This study documents B. burgdorferi infection in Mexican patients. Relevant epidemiologic data are 1) cases occurred after visiting forest areas, 2) patients reported having been bitten by a nonflying insect, 3) cases occurred during the summer-fall season, 4) no patient reported having traveled to another country, and 5) all skin lesions resolved after treatment with an antimicrobial drug. Our results suggest that B. burgdorferi infection occurs in Mexico and that continuous surveillance for Lyme disease in Mexico should be mandatory.
Dr Gordillo-Pérez is an associate researcher in the Infectious Diseases Research Unit at the Paediatric Hospital, Centro Médico Nacional Siglo XXI, IMSS, in Mexico City. Her research interests are diagnosis and epidemiology of Lyme borreliosis in Mexico, molecular typing of B. burgdorferi and its vectors, and infections of the central nervous system.
Acknowledgments
We thank C. Barthel for her excellent technical assistance.
Financial support was provided by the Consejo Nacional de Ciencia y Tecnología, Mexico (grant 30694-M) and by the Coordinación de Investigación en Salud, Instituto Mexicano del Seguro Social (IMSS), grant FP-2003/119, México. J.T. has a Fundacion-IMSS exclusivity scholarship.
References
- Centers for Disease Control and Prevention. Lyme Disease—United States, 2001–2002. MMWR Morb Mortal Wkly Rep. 2004;53:365–9.PubMedGoogle Scholar
- Rath PM, Ibershoff B, Mohnhaupt A, Albig J, Eljaschewitsch B, Jurgens D, Seroprevalence of Lyme borreliosis in forestry workers from Brandenburg, Germany. Eur J Clin Microbiol Infect Dis. 1996;15:372–7. DOIPubMedGoogle Scholar
- Gordillo G, Torres J, Solorzano F, Cedillo-Rivera R, Tapia-Conyer R, Muñoz O. Serological evidences suggesting the presence of Borrelia burgdorferi infection in Mexico. Arch Med Res. 1999;30:64–8. DOIPubMedGoogle Scholar
- Gordillo-Perez G, Torres J, Solorzano-Santos F, Garduño-Bautista V, Tapia-Conyer R, Muñoz O. Seroepidemiologic study of Lyme's borreliosis in Mexico City and the northeast of the Mexican Republic [in Spanish]. Salud Publica Mex. 2003;45:351–5. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the second national conference on serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 1995;44:590–1.PubMedGoogle Scholar
- Stanek G, O’Connell S, Cimmino M, Aberer E, Kristoferitsch W, Granström et al. European Union concerted action on risk assesment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–7.PubMedGoogle Scholar
- Hunfeld KP, Ernst M, Zachary P, Jaulhac B, Sonneborn HH, Brade V. Development and laboratory evaluation of a new recombinant ELISA for the serodiagnosis of Lyme disease. Wien Klin Wochenschr. 2002;114:580–5.PubMedGoogle Scholar
- Jaulhac B, Chary-Valckenaere I, Sibilia J, Javier RM, Piémont Y, Kuntz JL, Detection of Borrelia burgdorferi by DNA amplification in synovial tissue samples from patients with Lyme arthritis. Arthritis Rheum. 1996;39:736–45. DOIPubMedGoogle Scholar
- Jaulhac B, Heller R, Limbach FX, Hansmann Y, Lipsker D, Monteil H, Direct molecular typing of Borrelia burgdorferi sensu lato species in synovial samples from patients with Lyme arthritis. J Clin Microbiol. 2000;38:1895–900.PubMedGoogle Scholar
- Moter SE, Hofmann H, Wallich R, Simon M, Kramer M. Detection of Borrelia burgdorferi sensu lato in lesional skin of patients with erythema migrans and acrodermatitis chronica atrophicans by OspA–specific PCR. J Clin Microbiol. 1994;32:2980–8.PubMedGoogle Scholar
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–6. DOIPubMedGoogle Scholar
- Colli C, Leinweber B, Müllegger R, Chott A, Kerl H, Cerroni L. Borrelia burgdorferi–associated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232–40. DOIPubMedGoogle Scholar
- Picken RN, Strle F, Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, Cimperman J, Molecular subtyping of Borrelia burgdorferi sensu lato isolates from five patients with solitary lymphocytoma. J Invest Dermatol. 1997;108:92–7. DOIPubMedGoogle Scholar
- Busch U, Hizo-Teufel C, Böhmer R, Fingerle V, Rössler D, Wilske B, Borrelia burgdorferi sensu lato strains isolated from cutaneous Lyme borreliosis biopsies differentiated by pulsed-field gel electrophoresis. Scand J Infect Dis. 1996;28:583–9. DOIPubMedGoogle Scholar
- Finkel MF, Johnson RC. Borrelia lymphocytoma: a possible North American case. Wis Med J. 1990;89:683–6.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 13, Number 10—October 2007
| EID Search Options |
|---|
|
|
|
|
|
|