Volume 14, Number 5—May 2008
Dispatch
Bacteremia Caused by Group G Streptococci, Taiwan
Abstract
A retrospective observational study in Taiwan, 1998–2004, identified 92 patients with group G streptococcal bacteremia; 86 had Streptococcus dysgalactiae subspecies equisimilis. The most common diagnosis was cellulitis (48 cases), followed by primary bacteremia (34 cases). Infection recurred in 9 patients. Mortality rate was low (3.3%); resistance to quinupristin-dalfopristin was high.
Group G streptococci (GGS) are part of the normal microbial flora of the gastrointestinal tract, vagina, and skin and cause a variety of infections (1). Major underlying illnesses in patients with GGS bacteremia are malignancy, cardiovascular disease, diabetes mellitus, bone and joint diseases, and cirrhosis (1,2). Reported mortality rates for patients with GGS bacteremia also vary, ranging from 5% to 30% (1–3). Recent studies of β-hemolytic streptococci isolates carrying Lancefield group G antigen showed that they consist of Streptococcus dysgalactiae subspecies equisimilis, S. anginosus, and S. canis (2,4–6). To supplement the limited clinical information about bacteremia caused by GGS strains identified to the species level (2–4), we conducted a retrospective observational study.
We included all patients with GGS-positive blood cultures who had been treated from April 1998 through August 2004 at National Taiwan University Hospital, a 2,000-bed teaching hospital in northern Taiwan. We recorded demographic parameters, underlying illness, clinical diagnosis, and outcome for each patient. Clinical diagnosis was based on the attending physician’s judgment and examination results. Recurrence of bacteremia was defined as repeated positive blood culture after complete treatment (at least 14 days) of previous bacteremia.
Differentiation of GGS was based on colony size, hemolytic reaction, Voges-Proskauer reaction, and β-glucuronidase activity. All β-hemolytic streptococci, whether large or small colonies, were tested for Lancefield group by using an agglutination kit (Streptex; Murex Biotech Ltd., Dartford, UK). PCR to differentiate between S. anginosus and S. dysgalactiae subsp. equisimilis was performed for all GGS isolates as described (7). For identification of S. canis, a probable isolate was identified by a negative β-glucuronidase result and further confirmed with the 16sRNA method as described (8). Susceptibilities of these isolates were tested by using the broth microdilution method as defined by the Clinical and Laboratory Standards Institute (formerly National Committee for Clinical Laboratory Standards) (9).
To determine the similarity of isolates in cases of recurrence, we used pulsed-field gel electrophoresis (PFGE) as described (10). The emm typing of isolates in cases of recurrence were also determined as described (11). The first 160 bases sequenced by emmseq2 that had >95% identity were defined as having the same genotype (11).
During the study period, 106 episodes of GGS bacteremia in 92 patients had been recorded; 56 episodes occurred during the first half of the study period (before June 2001) and 50 episodes during the second half. The causative agent was S. dysgalactiae subsp. equisimilis for 99 episodes, S. anginosus for 5, and S. canis for 2. Bacteremia recurred for 9 patients (1 had 4 episodes, and 3 had 3 episodes); bacteremia was nosocomial for 7 patients and polymicrobial for 5. The clinical characteristics of the patients are summarized in Table 1. All 3 patients who died had a diagnosis of the primary bacteremia caused by S. dysgalactiae subsp. equisimilis.
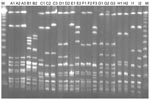
Among the 9 patients with recurrent bacteremia, the causative agent was S. dysgalactiae subsp. equisimilis for 8 and S. canis for 1. PFGE performed with all 13 available isolates from recurrent cases showed that 10 were identical to that of the initial episode, including 1 in a patient with recurrence of S. canis bacteremia. Sequence typing showed emm type stG485 for 4 patients. The clinical characteristics of the patients and emm typing results are shown in Table 2; PFGE results are shown in the Figure. The underlying diseases of patients with recurrent episodes included genital cancer (4 [44.4%] patients) and history of cellulitis (6 [66.7%]), each of which was significantly correlated with the likelihood of recurrence (p<0.01 for each). Further analysis showed that a previous history of cellulitis was significantly correlated with female sex (p = 0.01), genital cancer (p<0.01), tissue edema (p = 0.02), heart disease (p = 0.04), and post–coronary artery bypass graft (p = 0.03).
Bacteremia caused by β-hemolytic S. anginosus with group G antigen was identified for 5 patients, none of whom had cellulitis, compared with 48 (55.8%) of the 86 patients with S. dysgalactiae subsp. equisimilis who did have cellulitis (p = 0.03). Polymicrobial bacteremia and nosocomial bacteremia were found in a higher percentage of patients with S. anginosus (60% and 40.0%, respectively) than of patients with S. dysgalactiae subsp. equisimilis bacteremia (4.7% and 5.8%, respectively); p<0.01 and p = 0.02, respectively. The 1 patient with S. canis bacteremia was a 33-year-old man with no history of dog bite. He had alcohol-associated liver cirrhosis of Child C (severe) classification and leg edema. He had 2 episodes of S. canis bacteremia 1 month apart. Echocardiogram results showed no evidence of valvular vegetation. For the first episode, the patient received a 14-day course of cefotaxime.
Antimicrobial drug–susceptibility testing showed decreased susceptibility to only macrolides (susceptibity rates: azithromycin 67.4%, clarithromycin 73.9%), clindamycin (87.0%), and quinupristin-dalfopristin (33.7%) (Appendix Table). No clinical factor correlated with macrolide resistance. All isolates of recurrent bacteremia were susceptible to macrolides.
We documented 5 cases of primary bacteremia caused by β-hemolytic group G S. anginosus and unintentionally documented recurrence of S. canis bacteremia. S. canis bacteremia in humans was first clearly described in 1997 (12).
Our finding of 5 β-hemolytic S. anginosus isolates and 1 S. canis isolate in patients with GGS bacteremia in this study differs from findings of previous studies (2,3). Factors that may have contributed to this discrepancy include serotype determination and PCR method. Serotype determination was performed for all β-hemolytic streptococci isolated in our hospital, whether colonies were large or small, which might have led to the detection of more streptococcal isolates with G antigen. The PCR method developed in our hospital and used in this study could effectively differentiate S. anginosus from S. dysgalactiae subsp. equisimilis (7).
Information about clinical infection with S. milleri with group G antigen is limited (4). In a previous study of GGS bacteremia, Cohen-Poradosu et al. reported that 6 of 84 patients had recurrence of bacteremia (3). We found recurrence in 9 of the 92 patients. Risk factors were similar to those previously reported for non–group A streptococcal cellulitis (13). PFGE of these isolates showed that a high percentage of recurrence was caused by identical strains. Although Cohen-Poradosu et al. reported that emm type stG840 was the most common strain (3), we found emm type stG485 to be most common.
For years in Taiwan, macrolide resistance of streptococci has been a major health problem (14,15). A previous study found erythromycin resistance in 23.5% of GGS strains (14). Although we did not test for erythromycin resistance, we found some resistance even to new macrolides. Since restriction of macrolide use in Taiwan, a linear relationship has been noted between the decline in erythromycin use and the decline in erythromycin resistance in S. pyogenes (15). Our study, however, found no decline in macrolide resistance from first half of the study period (27.1%) to the second half (37.0%).
In summary, in our study, infection with S. dysgalactiae subsp. equisimilis was the most common cause of GGS bacteremia. Infection recurred for ≈10%. The mortality rate for patients with GGS bacteremia was relatively low (<10%), but resistance to quinupristin-dalfopristin was extremely high.
Dr Liao is an infectious diseases specialist at the Department of Internal Medicine, Far-Eastern Memorial Hospital. His major research interests are clinical and epidemiologic studies and pathogenesis of gram-positive bacterial infections, particularly streptococcal and methicillin-resistant Staphylococcus aureus infections.
References
- Auckenthaler R, Hermans PE, Washington JA II. Group G streptococcal bacteremia: clinical study and review of the literature. Rev Infect Dis. 1983;5:196–204.PubMedGoogle Scholar
- Woo PC, Fung AM, Lau SK, Wong SS, Yuen KY. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J Clin Microbiol. 2001;39:3147–55. DOIPubMedGoogle Scholar
- Cohen-Poradosu R, Jaffe J, Lavi D, Grisariu-Greenzaid S, Nir-Paz R, Valinsky L, Group G streptococcal bacteremia in Jerusalem. Emerg Infect Dis. 2004;10:1455–60.PubMedGoogle Scholar
- Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–30. DOIPubMedGoogle Scholar
- Vandamme P, Pot B, Falsen E, Kersters K, Devriese LA. Taxonomic study of Lancefield streptococcal groups C, G, and L (Streptococcus dysgalactiae) and proposal of S. dysgalactiae subsp. equisimilis subsp. nov. Int J Syst Bacteriol. 1996;46:774–81.PubMedGoogle Scholar
- Lawrence J, Yajko DM, Hadley WK. Incidence and characterization of beta-hemolytic Streptococcus milleri and differentiation from S. pyogenes (group A), S. equisimilis (group C), and large-colony group G streptococci. J Clin Microbiol. 1985;22:772–7.PubMedGoogle Scholar
- Liu LC, Tsai JC, Hsueh PR, Teng LJ. Rapid differentiation between members of the anginosus group and Streptococcus dysgalactiae subsp. equisimilis within beta-hemolytic group C and G streptococci by PCR. J Clin Microbiol. 2006;44:1836–8. DOIPubMedGoogle Scholar
- Whatmore AM, Engler KH, Gudmundsdottir G, Efstratiou A. Identification of isolates of Streptococcus canis infecting humans. J Clin Microbiol. 2001;39:4196–9. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A6. 5th ed. Wayne (PA): The Committee, 2004.
- Hsueh PR, Teng LJ, Lee LN, Yang PC, Ho SW, Luh KT. Dissemination of high-level penicillin–, extended-spectrum cephalosporin–, and erythromycin–resistant Streptococcus pneumoniae clones in Taiwan. J Clin Microbiol. 1999;37:221–4.PubMedGoogle Scholar
- Facklam R, Beall B, Efstratiou A, Fischetti V, Johnson D, Kaplan E, emm typing and validation of provisional M types for group A streptococci. Emerg Infect Dis. 1999;5:247–53.PubMedGoogle Scholar
- Bert F, Lambert-Zechovsky N. Septicemia caused by Streptococcus canis in a human. J Clin Microbiol. 1997;35:777–9.PubMedGoogle Scholar
- Baddour LM, Bisno AL. Non-group A beta-hemolytic streptococcal cellulitis. Association with venous and lymphatic compromise. Am J Med. 1985;79:155–9. DOIPubMedGoogle Scholar
- Wu JJ, Lin KY, Hsueh PR, Liu JW, Pan HI, Sheu SM. High incidence of erythromycin-resistant streptococci in Taiwan. Antimicrob Agents Chemother. 1997;41:844–6.PubMedGoogle Scholar
- Hsueh PR, Shyr JM, Wu JJ. Changes in macrolide resistance among respiratory pathogens after decreased erythromycin consumption in Taiwan. Clin Microbiol Infect. 2006;12:296–8. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 14, Number 5—May 2008
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Po-Ren Hsueh, National Taiwan University, No. 7, Chung-Shan South Road, 100 Taipei, Taiwan;
Top