Volume 16, Number 3—March 2010
Perspective
Potential for Tick-borne Bartonelloses
Abstract
As worldwide vectors of human infectious diseases, ticks are considered to be second only to mosquitoes. Each tick species has preferred environmental conditions and biotopes that determine its geographic distribution, the pathogens it vectors, and the areas that pose risk for tick-borne diseases. Researchers have identified an increasing number of bacterial pathogens that are transmitted by ticks, including Anaplasma, Borrelia, Ehrlichia, and Rickettsia spp. Recent reports involving humans and canines suggest that ticks should be considered as potential vectors of Bartonella spp. To strengthen this suggestion, numerous molecular surveys to detect Bartonella DNA in ticks have been conducted. However, there is little evidence that Bartonella spp. can replicate within ticks and no definitive evidence of transmission by a tick to a vertebrate host.
Bartonella spp. are gram-negative bacilli or coccobacilli that belong to the α-2 subgroup of Proteobacteria. According to 16S rDNA gene comparisons, they are closely related to the genera Brucella and Agrobacterium (1). A remarkable feature of the genus Bartonella is the ability of a single species to cause either acute or chronic infection that can cause either vascular proliferative lesions or suppurative and granulomatous inflammation. The pathologic response to infection with Bartonella spp. varies substantially with the status of the host’s immune system; vasoproliferative lesions are most frequently reported for immunocompromised patients. To date, 13 Bartonella species and subspecies have been associated with an increasing spectrum of clinical syndromes in humans, including cat-scratch disease and chronic bacteremia (B. henselae), bacillary angiomatosis (B. henselae, B. quintana), peliosis hepatitis (B. henselae), bacteremia and/or endocarditis (B. henselae, B. quintana, B. elizabethae, B. vinsonii subsp. arupensis, B. vinsonii subsp. berkhoffii, B. koehlerae, and B. alsatica), Carrión disease (B. bacilliformis), trench fever (B. quintana), retinitis and uveitis (B. henselae, B. grahamii), myocarditis (B. vinsonii subsp. berkhoffii, B. washoensis), splenomegaly (B. bacilliformis, B. henselae, B. rochalimae), and fever and fatigue (B. henselae, B. vinsonii subsp. berkhoffii, B. tamiae) (1–3).
Ticks were first identified as potential vectors of Babesia bigemina, the agent of Texas cattle fever, in 1893 (4). There are 2 major tick families (≈865 tick species worldwide): the Ixodidae, or hard ticks, characterized by a sclerotized dorsal plate, and the Argasidae, or soft ticks, characterized by their flexible cuticle. A third family, the Nuttalliellidae, is represented by a single species that is confined to southern Africa. The genus Ixodes, family Ixodidae, contains >200 species, of which 14 make up the I. ricinus complex (4). Among these 14 species, I. scapularis, I. pacificus, I. ricinus, and I. persulcatus ticks are involved in the transmission of the Borrelia burgdorferi complex, which is a prevalent cause of Lyme disease in persons in the Northern Hemisphere.
Ticks in various regions of the world are vectors for bacterial, viral, and protozoal pathogens (5). Ticks may act not only as vectors but also as reservoirs of tick-transmitted bacteria that are transmitted transstadially and transovarially in a tick species (e.g., certain Rickettsia spp. and Borrelia spp.) (5). When feeding on an infected small-mammal host, larvae and nymphs can ingest >1 pathogens while obtaining a blood meal. Some organisms are then passaged to the next stage in the tick life cycle and can be transmissible during the subsequent blood meal (5). For each tick species, the optimal environmental conditions determine the geographic distribution; the spectrum of tick-borne pathogens; and as a result, the geographic areas of risk for tick-borne diseases, particularly when ticks are both vectors and reservoirs of specific pathogens.
Hard ticks are the primary vectors of a variety of bacterial pathogens, including Anaplasma spp., Borrelia spp., Ehrlichia spp., Coxiella burnetii, and Rickettsia spp (5–7). Anaplasma phagocytophilum is transmitted by I. persulcatus–complex ticks, including I. scapularis, I. pacificus, and I. ricinus, whereas Ehrlichia chaffeensis and Ehrlichia ewingii are transmitted by Amblyomma americanum ticks (5,6). Although some pathogens are carried by a single or limited number of tick species, other organisms such as Coxiella burnetii have been identified in >40 tick species (7). Lyme disease, caused by B. burgdorferi, is transmitted by I. scapularis and I. pacificus ticks within the United States, by I. ricinus ticks in Europe, and by other Ixodes spp. ticks in the Northern Hemisphere (5,8). Although specific Bartonella spp. are transmitted by blood-sucking arthropods, including fleas, lice, or sandflies, the only evidence to support the possibility of tick-borne transmission is indirect.
We present an overview of the various Bartonella spp. that have been detected in ticks and discuss human cases of Bartonella infection that are suggestive of tick transmission. Because of the rapidly expanding number of reservoir host–adapted Bartonella spp. that have been discovered in recent years, efforts to clarify modes of transmission are relevant to public health in terms of interrupting the transmission process. As evolving evidence supports the ability of this genus to induce chronic intravascular infections in humans, improved understanding of vector competence could facilitate efforts to block pathogen transmission, which would help improve human health (9).
Bartonella spp. have a natural cycle of chronic intravascular infection in a reservoir host and a sustained pattern of bacterial transmission by a defined and evolutionarily well-adapted vector from the reservoir hosts to new susceptible hosts. Current information leads to the presumption of a long-standing and highly adapted species-specific association between a given Bartonella sp. and the preferred animal host and vector (10). Inadvertent infection of persons with at least 13 Bartonella spp. has resulted in a wide spectrum of disease manifestations. After primary infection of the natural mammalian host, a chronic, relapsing, nonclinical bacteremia occurs. At times, in wild and stray animal populations, including cats, cows, and various rodent species, the prevalence of infection within the population can approach 100% (1). Although the geographic distribution of a specific Bartonella sp. may reflect the geographic distribution of its hosts or vectors, knowledge related to vector transmission of Bartonella organisms remains inadequate.
Bartonella spp. DNA in Ticks
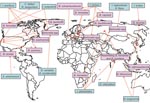
As an initial effort to define tick species that might serve as competent vectors for transmission of Bartonella spp., molecular epidemiology surveys to identify Bartonella spp. DNA in ticks have been conducted (2). Bartonella spp. have mostly been identified by PCR using primers targeting either specific Bartonella genes like the citrate synthase gene (gltA) gene, the riboflavin synthase gene, the heat shock protein gene (groEL), the 16S–23S intergenic spacer, the heme binding protein gene, and the cell division protein gene or the 16S rDNA gene (Table 1). Summarized results indicate that the proportion of ticks harboring Bartonella DNA can vary from low prevalences of 0.43% among questing A. americanum ticks examined in the southeastern United States (3) and 1.2% of I. ricinus ticks collected in the Czech Republic (24) to a prevalence as high as 60% in I. ricinus ticks from roe deer in the Netherlands (20) (Table 1). Bartonella spp. from various locations tend to differ. For example, Bartonella DNA related to B. doshiae, B. rattimassiliensis, and B. tribocorum has been identified in ticks only in Asia, B. bacilliformis–like DNA and B. capreoli in ticks only in Europe, and B. washoensis, B. tamiae–like DNA, and B. vinsonii subsp. berkhoffii in ticks only in the United States (Figure).
Evidence for Co-infections in Ticks
In recent years, emphasis on the potential transmission of multiple pathogens by an individual tick after attachment to an animal or person has grown. While studying different tick populations throughout the world, several researchers have identified Bartonella DNA in conjunction with known tick-transmitted organisms. Adelson et al. tested for the prevalence of B. burgdorferi, Babesia microti, A. phagocytophilum, and Bartonella spp. in 107 I. scapularis ticks collected in New Jersey (27). A large percentage of ticks (45.8%) contained DNA from at least 1 of these organisms, and 34.5% of ticks screened harbored Bartonella spp. DNA. Of the ticks positive for Bartonella by PCR, 9 (8.4%) contained B. burgdorferi DNA, 1 (0.9%) contained B. microti DNA, 1 (0.9%) contained A. phagocytophilum DNA, 1 (0.9%) contained both B. burgdorferi and A. phagocytophilum DNA, and 1 (0.9%) contained B. microti and A. phagocytophilum DNA (27). Although the primers in this study were originally selected for the species-specific amplification of B. henselae, this region of the Bartonella 16S rDNA gene is highly conserved among many Bartonella spp. In a study performed in France, Halos et al. screened 92 questing I. ricinus ticks and determined that 9.8% contained Bartonella DNA by using gltA-specific primers (22). Bartonella schoenbuchensis–like DNA (96% homology) was detected in 1 of the adult ticks tested. The authors also reported that 1% of the ticks contained Bartonella spp. and B. burgdorferi DNA, 4% contained Bartonella and Babesia spp. DNA, and 1% contained Bartonella spp., B. burgdorferi, and Babesia spp. DNA (22). Of 168 questing adult I. pacificus ticks from Santa Cruz County, California, screened for Bartonella DNA, 11 (6.55%) contained B. henselae genotype I DNA (31). Of the Bartonella–positive ticks, 1.19% also harbored B. burgdorferi DNA and 2.98% harbored A. phagocytophilum DNA (31). Loftis et al. tested Carios kelleyi ticks, argasid tick species found on bats, from residential and community buildings in Iowa, for Anaplasma, Bartonella, Borrelia, Coxiella, and Rickettsia spp. One tick was found to contain Bartonella and Rickettsia DNA, and the DNA sequence was most closely related to B. henselae (11). Recently, Sun et al. examined Haemaphysalis longicornis and I. sinensis from People’s Republic of China for Borrelia, Bartonella, Anaplasma, and Erhlichia spp. (15). Of adult and nymphal H. longicornis ticks collected in the cities of Benxi and Liaoyang, 36% of 150 groups (60 individual host-associated adults, 30 pools of 2 questing adults, and 60 pools of 5 nymphs) harbored detectable Bartonella DNA. Furthermore, 16.3% of 86 individual I. sinensis ticks (all host-associated adults) from the cities of Tiantai, Jindong, and Jiangshan contained Bartonella DNA. One tick harbored all 4 bacteria (Borrelia, Bartonella, Anaplasma, and Ehrlichia spp. DNA), and a second tick pool was positive by PCR for Borrelia, Bartonella, and Ehrlichia spp (15).
Evidence of Potential Tick Bartonella spp. Transmission to Humans
In 1992, B. henselae infection developed in 2 previously healthy, immunocompetent men within weeks of a tick bite (32) (Table 2). Both patients reported signs and symptoms generally associated with B. henselae infection: fever, muscle and joint pain, headache, and photophobia. The first patient did not recall being bitten or scratched by a cat, the general mode of B. henselae transmission to humans. B. henselae organisms were cultured from the blood of both patients and confirmed by PCR. To our knowledge, this was the first case report to suggest that ticks may be responsible for transmission of Bartonella spp. in humans. More recently, B. henselae was isolated from a boy who had severe intractable migraine headaches 10 days after an attached tick was removed from his leg, although on the basis of seroconversion, infection with B. vinsonii subsp. berkhoffii was suspected (9). Breitschwerdt et al. concluded that the boy was either co-infected or chronically infected with B. henselae, the organism isolated, and subsequently infected with B. vinsonii subsp. berkhoffii, as reflected by the documentation of seroconversion.
In a clinical study, Zangwill et al. were interested in identifying risk factors associated with development of cat-scratch disease (33). The epidemiologic survey, performed in Connecticut, contained 56 cat-scratch disease patients and their controls (persons who owned or had been in contact with cats). They used a modified random-digit dialing technique to recruit controls, and they identified 60 patients with cat-scratch disease. However, of the 60 patients whose illnesses met the case definition, 4 were not successfully matched with controls for age and cat ownership; therefore, 56 patients and their controls were enrolled in the case–control study. The controls did not differ significantly from the patients by race, sex, family size, level of maternal education, or socioeconomic status. Answers to questionnaires suggested that cat-scratch disease was more likely to occur in patients than in controls if the person owned a kitten, had contact with a kitten with fleas, or had been bitten or scratched by a kitten. Of the 56 patients, 21% were also more likely than controls to have been bitten by a tick, although bivariate analysis did not demonstrate a significant association between tick bite and cat-scratch disease development (33).
Other case reports have suggested potential human co-infections with Bartonella spp. and a known tick-transmitted organism. Eskow et al. described 4 cases in which patients from central New Jersey reported several neurologic symptoms, including headache, fatigue, insomnia, and depression, which may have resulted from Lyme disease (caused by B. burgdorferi) (28). However, other causes for their cognitive dysfunctions cannot be ruled out. Of these 4 patients, 2 had histories of Lyme disease, and 3 had B. burgdorferi DNA in the cerebrospinal fluid (CSF). One patient exhibited no laboratory evidence of Lyme disease, suggesting that these symptoms might have been caused by an agent other than B. burgdorferi. However, 2 patients reported illness within 1 week to 3 months after being bitten by a tick. Upon further investigation, all patients were seroreactive to B. henselae; immunofluorescence assay showed immunoglobulin (Ig) G titers of 64–256. According to the authors, B. henselae DNA was amplified from blood of 1 patient, from CSF of 1 patient, and from both blood and CSF of the other 2 patients (B. burgdorferi DNA also was detected in the CSF of these 2 patients). Ticks, identified as I. scapularis, found in 2 patients’ homes potentially harbored both B. henselae and B. burgdorferi DNA. Whether B. henselae was specifically detected in this case series is unclear because sequencing of amplicons was not performed and because the PCR primer set targeted the Bartonella 16S rRNA, a highly conserved region. Without sequencing of amplicons or confirmation of results by targeting a more highly variable gene, ascertaining whether B. henselae was present in the ticks or in the patients would be difficult. However, the results derived from these cases are of interest because, to our knowledge, this was the first case series to propose simultaneous detection of both B. burgdorferi and Bartonella DNA in the CSF of patients with neurologic signs.
In another study, 2 of 17 patients from Poland with symptoms suggestive of neuroborreliosis seemed to be co-infected with B. burgdorferi and B. henselae (34). B. burgdorferi–specific antibodies were detected in a patient whose CSF also had detectable B. henselae DNA. The other patient was seroreactive to both B. burgdorferi and B. henselae antigens at titers of 32. The authors speculated that co-infection may be tick transmitted; however, contact with other arthropod species should be considered. Although the detection of B. henselae DNA in the CSF of these patients could be attributed to amplification of DNA from nonviable organisms or to laboratory error, the repeated documentation of B. henselae in blood and in CSF of a young woman with a previous diagnosis of classical cat-scratch disease support the potential that this bacterium can cause chronic intravascular and central nervous system infections in immunocompetent persons (9).
In a study performed in Slovenia, 86 febrile children were screened for serologic evidence of exposure to multiple tick-borne organisms within 6 weeks of a known tick bite (35). Acute- and convalescent-phase serum samples were collected from each child. Prior exposure was determined for 5 children who harbored B. henselae IgG and for 4 children who harbored B. quintana IgG. Seroconversion of IgG to both antigens was detected for only 1 child (35). Morozova et al. tested for Bartonella DNA in persons from the Novosibirsk region of Russia who had been bitten by ticks during the summers of 2003 and 2004 (38). Bartonella DNA closely related to B. henselae and B. quintana was detected in the blood of some patients by using groEL-specific primers (36). A more recent study, performed by Breitschwerdt et al., screened 42 immunocompetent patients, who had had prior animal and arthropod contact, for Bartonella spp. (37) The study included 12 women and 2 men who reported having had occupational animal contact for >10 years, including frequent animal bites, animal scratches, and arthropod exposure (e.g., fleas, ticks, biting flies, mosquitoes, lice, mites, chiggers). B. henselae or B. vinsonii subsp. berkhoffii were detected by PCR or were cultured from all patients (37). Case studies and surveys of this type suggest that ticks may serve as competent vectors of Bartonella spp., but this supposition cannot be confirmed until experimental studies demonstrating successful transmission have been performed.
Recently, Cotté et al. detailed the potential transmission of B. henselae by I. ricinus ticks (38). Using an artificial feeding platform made of rabbit skin, the authors successfully (based on PCR screening) infected ticks with B. henselae of molted ticks previously fed infected blood, suggesting that transstadial transmission may be possible. Subsequently, molted ticks were placed onto rabbit skins and fed noninfected blood, after which B. henselae was either cultured or detected by PCR analysis within 72 hours of when aliquots were taken from the previously noninfected blood. This finding indicates that during a blood meal, the organism could potentially be transferred from an infected tick to a noninfected individual. In addition, B. henselae bacteria were also present within molted ticks in sufficient numbers to cause bacteremia when tick salivary gland extracts were inoculated intravenously into domestic cats. Because ticks were not allowed to attach directly to the cats, this study supports, but does not prove, tick transmission of B. henselae by I. ricinus. Consistent with the transmission of Bartonella spp. by other arthropods such as fleas and lice, B. henselae does not seem to be transovarially transmitted in ticks because larvae hatched from B. henselae–positive (by PCR) egg clutches did not harbor detectable Bartonella DNA (2,38).
The number of zoonotic Bartonella spp. identified in the past 15 years has increased considerably. This review indicates that a diversity of Bartonella spp. DNA can be amplified from various tick species from numerous geographic locations, that tick attachment has preceded the onset of illness in a small number of patients from whom B. henselae DNA has been amplified, and that serologic and molecular evidence suggests cosegregation of Bartonella spp. with known tick-borne pathogens. Therefore, ticks might serve as potential Bartonella vectors. However, there is little evidence that Bartonella spp. can replicate within ticks and no definitive evidence of transmission by a tick to a vertebrate host. Only Kruszewska and Tylewska-Wiezbanowska reported successful isolation of Bartonella sp. from a tick (25); all other studies were based on amplification of Bartonella DNA from ticks by using PCR. As the medical relevance of the genus Bartonella continues to evolve, it is clearly necessary to determine whether ticks or other arthropods play a role in the transmission of Bartonella spp. among animals and humans. For this reason, experimental transmission studies, using infected ticks placed on live animals, are required to determine whether ticks are vector competent for the transmission of Bartonella spp.
Since the submission of this manuscript, we found 3 cases of B. henselae infection transmitted by Dermancentor spp. ticks. These patients had scalp eschar and neck lymphadenopathy (39).
Dr Angelakis is a clinician and researcher at the Unité des Rickettsies in Marseille. His research interests are zoonotic pathogens.
References
- Rolain JM, Brouqui P, Koehler JE, Maguina C, Dolan MJ, Raoult D. Recommendations for treatment of human infections caused by Bartonella species. Antimicrob Agents Chemother. 2004;48:1921–33. DOIPubMedGoogle Scholar
- Billeter SA, Levy MG, Chomel BB, Breitschwerdt EB. Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Med Vet Entomol. 2008;22:1–15. DOIPubMedGoogle Scholar
- Billeter SA, Miller MK, Breitschwerdt EB, Levy MG. Detection of two Bartonella tamiae–like sequences in Amblyomma americanum (Acari: Ixodidae) using 16S–23S intergenic spacer region–specific primers. J Med Entomol. 2008;45:176–9. DOIPubMedGoogle Scholar
- Swanson SJ, Neitzel D, Reed KD, Belongia EA. Coinfections acquired from Ixodes ticks. Clin Microbiol Rev. 2006;19:708–27. DOIPubMedGoogle Scholar
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897–928. DOIPubMedGoogle Scholar
- Inokuma H. Vector and reservoir hosts of Anaplasmataceae. In: Rickettsial diseases. Infectious Diseases and Therapy Series. 2007;42:199–212.
- Hildenbrand P, Craven DE, Jones R, Nemeskal P. Lyme neuroborreliosis: manifestations of a rapidly emerging zoonosis. AJNR Am J Neuroradiol. 2009;30:1079–87. DOIPubMedGoogle Scholar
- Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, Woods CW. Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol. 2008;46:2856–61. DOIPubMedGoogle Scholar
- Houpikian P, Raoult D. Molecular phylogeny of the genus Bartonella: what is the current knowledge? FEMS Microbiol Lett. 2001;200:1–7. DOIPubMedGoogle Scholar
- Loftis AD, Gill JS, Schriefer ME, Levin ML, Eremeeva ME, Gilchrist MJ, Detection of Rickettsia, Borrelia, and Bartonella in Carios kelleyi (Acari: Argasidae). J Med Entomol. 2005;42:473–80. DOIPubMedGoogle Scholar
- Chang CC, Hayashidani H, Pusterla N, Kasten RW, Madigan JE, Chomel BB. Investigation of Bartonella infection in ixodid ticks from California. Comp Immunol Microbiol Infect Dis. 2002;25:229–36. DOIPubMedGoogle Scholar
- Rar VA, Fomenko NV, Dobrotvorsky AK, Livanova NN, Rudakova SA, Fedorov EG, Tickborne pathogen detection, western Siberia, Russia. Emerg Infect Dis. 2005;11:1708–15.PubMedGoogle Scholar
- Kim CM, Kim JY, Yi YH, Lee MJ, Cho MR, Shah DH, Detection of Bartonella species from ticks, mites and small mammals in Korea. J Vet Sci. 2005;6:327–34.PubMedGoogle Scholar
- Sun J, Liu Q, Lu L, Ding G, Guo J, Fu G, Coinfection with four genera of bacteria (Borrelia, Bartonella, Anaplasma, and Ehrlichia) in Haemaphysalis longicornis and Ixodes sinensis ticks from China. Vector Borne Zoonotic Dis. 2008;8:791–5. DOIPubMedGoogle Scholar
- Chang CC, Chomel BB, Kasten RW, Romano V, Tietze N. Molecular evidence of Bartonella spp. in questing adult Ixodes pacificus ticks in California. J Clin Microbiol. 2001;39:1221–6. DOIPubMedGoogle Scholar
- Morozova OV, Cabello FC, Dobrotvorsky AK. Semi-nested PCR detection of Bartonella henselae in Ixodes persulcatus ticks from western Siberia, Russia. Vector Borne Zoonotic Dis. 2004;4:306–9. DOIPubMedGoogle Scholar
- Sanogo YO, Zeaiter Z, Caruso G, Merola F, Shpynov S, Brouqui P, Bartonella henselae in Ixodes ricinus ticks (Acari: Ixodida) removed from humans, Belluno Province, Italy. Emerg Infect Dis. 2003;9:329–32.PubMedGoogle Scholar
- Podsiadly E, Chmielewski T, Sochon E, Tylewska-Wierzbanowska S. Bartonella henselae in Ixodes ricinus ticks removed from dogs. Vector Borne Zoonotic Dis. 2007;7:189–92. DOIPubMedGoogle Scholar
- Schouls LM, Van de Pol I, Rijpkema SGT, Schot CS. Detection and identification of Ehrlichia, Borrelia burgdorferi sensu lato, and Bartonella species in Dutch Ixodes ricinus ticks. J Clin Microbiol. 1999;37:2215–22.PubMedGoogle Scholar
- Schabereiter-Gurtner C, Lubitz W, Rolleke S. Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria. J Microbiol Methods. 2003;52:251–60. DOIPubMedGoogle Scholar
- Halos L, Jamal T, Maillard R, Beugnet F, Le Menach A, Boulouis HJ, Evidence of Bartonella sp. in questing adult and nymphal Ixodes ricinus ticks from France and co-infection with Borrelia burgdorferi sensu lato and Babesia sp. Vet Res. 2005;36:79–87. DOIPubMedGoogle Scholar
- Bogumila S, Adamska M. Capreolus capreolus and Ixodes ricinus as a reservoir of Bartonella in northwestern Poland [in Polish]. Wiad Parazytol. 2005;51:139–43.PubMedGoogle Scholar
- Hercík K, Hásová V, Janecek J, Branny P. Molecular evidence of Bartonella DNA in ixodid ticks in Czechia. Folia Microbiol (Praha). 2007;52:503–9. DOIPubMedGoogle Scholar
- Kruszewska D, Tylewska-Wierzbanowska S. Unknown species of rickettsiae isolated from Ixodes riconus tick in Walcz. Rocz Akad Med Bialymst. 1996. 1996;41:129–135.
- Matsumoto K, Berrada ZL, Klinger E, Goethert HK, Telford SR III. Molecular detection of Bartonella schoenbuchensis from ectoparasites of deer in Massachusetts. Vector Borne Zoonotic Dis. 2008;8:549–54. DOIPubMedGoogle Scholar
- Adelson ME, Rao RV, Tilton RC, Adelson ME, Rao RV, Tilton RC, Prevalence of Borrelia burgdorferi, Bartonella spp., Babesia microti, and Anaplasma phagocytophila in Ixodes scapularis ticks collected in northern New Jersey. J Clin Microbiol. 2004;42:2799–801. DOIPubMedGoogle Scholar
- Eskow E, Rao RV, Mordechai E. Concurrent infection of the central nervous system by Borrelia burgdorferi and Bartonella henselae: evidence for a novel tick-borne disease complex. Arch Neurol. 2001;58:1357–63. DOIPubMedGoogle Scholar
- Wikswo ME, Hu R, Metzger ME, Eremeeva ME. Detection of Rickettsia rickettsii and Bartonella henselae in Rhipicephalus sanguineus ticks from California. J Med Entomol. 2007;44:158–62. DOIPubMedGoogle Scholar
- Parola P, Shpynov S, Montoya M, Lopez M, Houpikian P, Zeaiter Z, First molecular evidence of new Bartonella spp. in fleas and a tick from Peru. Am J Trop Med Hyg. 2002;67:135–6.PubMedGoogle Scholar
- Holden K, Boothby JT, Kasten RW, Chomel BB. Co-detection of Bartonella henselae, Borrelia burgdorferi, and Anaplasma phagocytophilum in Ixodes pacificus ticks from California, USA. Vector Borne Zoonotic Dis. 2006;6:99–102. DOIPubMedGoogle Scholar
- Lucey D, Dolan MJ, Moss CW, Garcia M, Hollis DG, Wegner S, Relapsing illness due to Rochalimaea henselae in immunocompetent hosts: implication for therapy and new epidemiological associations. Clin Infect Dis. 1992;14:683–8.PubMedGoogle Scholar
- Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, Plikaytis BD, Hadler JL, Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. DOIPubMedGoogle Scholar
- Podsiadly E, Chmielewski T, Tylewska-Wierzbanowska S. Bartonella henselae and Borrelia burgdorferi infections of the central nervous system. Ann N Y Acad Sci. 2003;990:404–6. DOIPubMedGoogle Scholar
- Arnez M, Luznik-Bufon T, Avsic-Zupanc T, Ruzic-Sabljic E, Petrovec M, Lotric-Furlan S, Causes of febrile illnesses after a tick bite in Slovenian children. Pediatr Infect Dis J. 2003;22:1078–83. DOIPubMedGoogle Scholar
- Morozova OV, Chernousova NI, Morozov IV. Detection of the Bartonella DNA by the method of nested PCR in patients after tick bites in Novosibirsk region [in Russian]. Mol Gen Mikrobiol Virusol. 2005;4:14–7.PubMedGoogle Scholar
- Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, Hegarty BC, Woods CW. Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis. 2007;13:938–41.PubMedGoogle Scholar
- Cotté V, Bonnet S, Le-Rhun D, Le Naour E, Chauvin A, Boulouis HJ, Transmission of Bartonella henselae by Ixodes ricinus. Emerg Infect Dis. 2008;14:1074–80. DOIPubMedGoogle Scholar
- Angelakis E, Pulcini C, Waton J, Imbert P, Socolovschi C, Edouard S, Scalp eschar and neck lymphadenopathy caused by Bartonella henselae after tick bite. Clin Infect Dis. 2010 Jan 13; [Epub ahead of print].
Figure
Tables
Cite This ArticleTable of Contents – Volume 16, Number 3—March 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Address for correspondence Didier Raoult, Unité des Rickettsies, CNRS UMR 6020, IFR 48, Faculté de Médecine, Université de la Méditerranée, 27 Blvd Jean Moulin, 13385 Marseille CEDEX 05, France
Top