Volume 16, Number 4—April 2010
Dispatch
Plasmodium knowlesi in Human, Indonesian Borneo
Abstract
Plasmodium knowlesi is now established as the fifth Plasmodium species to cause malaria in humans. We describe a case of P. knowlesi infection acquired in Indonesian Borneo that was imported into Australia. Clinicians need to consider this diagnosis in a patient who has acquired malaria in forest areas of Southeast Asia.
Plasmodium knowlesi is now recognized as a cause of potentially fatal human malaria in forest areas of Southeast Asia. We describe a case of P. knowlesi malaria acquired in Indonesia and imported to Australia.
A 39-year-old man from Australia came to a suburban hospital in Sydney, New South Wales, Australia, with a 2-week history of morning fevers and mild headaches. His symptoms started 13 days after he left Indonesian Borneo (Kalimantan). The patient had spent an average of 10 days per month for the past 18 months working adjacent to a forest area in South Kalimantan Province, Indonesian Borneo. The most recent visit was toward the end of the rainy season. He did not use any personal vector avoidance measures (mosquito nets, long clothing, insect repellent) or receive malaria chemoprophylaxis. The patient did not travel to any other malaria-endemic areas during this 18-month period.
He did not have a remarkable medical history. On examination, he was febrile (38.9°C) and had a heart rate of 88 beats/min, blood pressure of 128/78 mm Hg, normal respiration rate, and oxygen saturation of 99% on room air. Physical examination was unremarkable. Laboratory investigations showed mild thrombocytopenia (106 × 109 cells/L, reference range 150–450 × 109 cells/L), mild leukopenia (3.7 × 109 cells/L, reference range 4.3–10 × 109 cells/L), and unremarkable results for levels of hemoglobin (142 g/L, reference range 130–180 g/L), bilirubin (12 μmol/L, reference value <20 μmol/L), and creatinine (95 μmol/L, reference range 40–120 μmol/L).
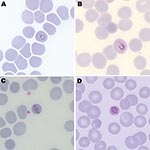
Malaria parasites were seen on Giemsa-stained thick and thin blood films (parasitemia level 185 parasites/μL). Parasite morphologic features resembled those of P. malariae with typical trophozoite band forms and heavily pigmented schizonts found inside smaller erythrocytes (Figure). Some parasites had morphologic features similar to those of P. falciparum. These similarities included ring forms and mature trophozoites with stippling of erythrocytes (Figure, panel B). A rapid diagnostic test result for histidine-rich protein 2 of P. falciparum was negative.
Given increased reports of P. knowlesi in Malaysian Borneo, we conducted molecular studies to identify the species. Results of multiplex PCRs for P. falciparum, P. vivax, P. malariae, and P. ovale were negative (1). Results of a PCR using P. knowlesi–specific primers (2) were positive for undiluted and diluted (1:50) blood samples. Sequencing of a small subunit rRNA gene product showed 100% identity with P. knowlesi (National Center for Biotechnology Information accession no. GU049678).
The patient was treated with atovaquone/proguanil, 250 mg/100 mg, 4×/day for 3 days. His fever resolved and the thrombocyte count returned to the reference level within 48 hours. He did not show any complications.
Naturally acquired human infection with P. knowlesi was first described in Malaysian Borneo in 1965 after an unusual sequence of events (3). Extensive investigation at this time failed to demonstrate zoonotic transmission of simian malaria to humans. More recently, molecular techniques have identified human infections with P. knowlesi, establishing it as the fifth Plasmodium species that infects humans (2). P. knowlesi accounts for most (70%) human malaria infections requiring hospitalization in Sarawak, Malaysian Borneo (4), and is widespread throughout Malaysia (5). Reports have also described human infections in Thailand (6), along the border of the People’s Republic of China and Myanmar (7), Singapore (8), and the Philippines (9). A recent epidemiologic study reported that 4/22 malaria cases diagnosed by microscopy as P. falciparum, P. vivax, or mixed P. falciparum/P. vivax infections were identified retrospectively by PCR to be mixed infections that included P. knowlesi (10). Presumably, P. knowlesi may account for a higher proportion of cases if those diagnosed morphologically as P. malariae were investigated.
Human malaria was considered to be caused by only 4 Plasmodium species in the premolecular biology era. Simian and human malaria parasites, including P. knowlesi and P. malariae, are often indistinguishable morphologically. Clues to identification of P. knowlesi by light microscopy that are useful, if present, include early trophozoites with fine ring forms, double chromatin dots, and 2–3 parasites per erythrocyte (resembling P. falciparum), trophozoites with a bird’s-eye appearance, mature trophozoites with a band appearance resembling P. malariae (Figure, panel C), and mature schizonts with a higher average merozoite count (16/erythrocyte) than in P. malariae (12/erythrocyte) (2,11).
Commercially available rapid diagnostic tests do not distinguish P. knowlesi from other forms of human malaria parasites. Lactate dehydrogenase produced by the 4 other Plasmodium spp. (pLDH) that cause human malaria is also present in P. knowlesi. Antibodies specific for pLDH of P. falciparum and P. vivax cross-react with pLDH of P. knowlesi (12) and therefore cannot be used to reliably distinguish P. knowlesi from mixed infections.
Distinction of P. knowlesi from P. malariae has useful management implications for patients and public health control measures. P. knowlesi potentially can cause severe disease and death, whereas P. malariae is generally benign. Daneshvar et al. recently published a prospective study of P. knowlesi infection in humans (4). They reported thrombocytopenia in 100% (107/107) of persons infected with P. knowlesi and lower mean ± SD thrombocyte counts (71 ± 35 × 109 cells/L) than in persons infected with P. falciparum (108 ± 59 × 109 cells/L) or P. vivax (118 ± 51 × 109 cells/L). Mean parasitemia level was 1,387 parasites/μL; 30.8% (33/107) of the case-patients had <500 parasites/μL. Severe infection was found in 7 (6.5%) of 107 patients, and the case-fatality rate was 1.8% (2/107) among hospitalized patients (4).
Deaths and severe disease caused by P. knowlesi result from pulmonary and hepatorenal failure (5). Severity of P. knowlesi infection is related to potentially high parasitemia levels produced by its rapid and unique 24-hour erythrocytic cycle and its ability to infect all stages of erythrocytes (13). Sequestration is not thought to occur during P. knowlesi infection, and neurologic complications seen during P. falciparum infection have not been described. Although our patient was treated with atovaquone/proguanil, patients with similar uncomplicated cases have responded well to treatment with chloroquine (4).
Public health control is challenging in areas where zoonotic human malaria is endemic (14). Standard public health measures for malaria prevention (insecticide-treated nets, indoor residual spraying, and intermittent preventive treatment in the reservoir population) are likely to be less effective than for typical forms of human malaria. Nevertheless, travelers to malaria-endemic areas should be encouraged to practice mosquito bite protection measures and chemoprophylaxis.
P. knowlesi malaria is transmitted from long-tailed (Macaca fasicularis) and pig-tailed (M. nemestrina) macaques to humans by Anopheles latens mosquitoes (in the Kapit Division of Malaysian Borneo) when humans visit forest or forest fringe areas. However, transmission does not seem to occur readily in villages (2,15). Increased recognition of P. knowlesi indicates that human infection is possible by with other simian malaria parasites (P. cynomolgi and P. inui).
We report a patient with P. knowlesi infection that was acquired in Indonesia and imported to Australia. Fortunately, this patient had a low parasitemia level and mild disease. A high degree of clinical suspicion is likely to increase the number of P. knowlesi cases diagnosed in patients with malaria acquired in forest areas of Southeast Asia.
Dr Figtree is microbiology registrar at Royal North Shore Hospital, Sydney. Her research interests include the molecular diversity of P. vivax.
Acknowledgment
We thank John Barnwell for providing P. knowlesi DNA used as PCR controls.
References
- Padley D, Moody AH, Chiodini PL, Saldanha J. Use of a rapid, single-round, multiplex PCR to detect malarial parasites and identify the species present. Ann Trop Med Parasitol. 2003;97:131–7. DOIPubMedGoogle Scholar
- Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–24. DOIPubMedGoogle Scholar
- Chin W, Contacos PG, Coatney GR, Kimball HR. A naturally acquired quotidian-type malaria in man transferable to monkeys. Science. 1965;149:865. DOIPubMedGoogle Scholar
- Daneshvar C, Davis TM, Cox-Singh J, Rafa’ee MZ, Zakaria SK, Divis PC, Clinical and laboratory features of human Plasmodium knowlesi infection. Clin Infect Dis. 2009;49:852–60. DOIPubMedGoogle Scholar
- Cox-Singh J, David TM, Lee KS, Shamsul SS, Matusop A, Ratnam S, Plasmodium knowlesi malaria in humans in widely distributed and potentially life threatening. Clin Infect Dis. 2008;46:165–71. DOIPubMedGoogle Scholar
- Jongwutiwes S, Putaporntip C, Takuya I, Tetsutaro S, Hiroji K. Naturally acquired Plasmodium knowlesi malaria in human, Thailand. Emerg Infect Dis. 2004;10:2211–3.PubMedGoogle Scholar
- Zhu HM, Li J, Zheng H. Human natural infection of Plasmodium knowlesi [in Chinese]. Zhongguo Ji Sheng Chong Xue Ji Sheng Chong Bing Za Zhi. 2006;24:70–1.
- Ng OT, Ooi EE, Lee CC, Lee PJ, Ng LC, Wong PS, Naturally acquired human Plasmodium knowlesi infection, Singapore. Emerg Infect Dis. 2008;14:814–6. DOIPubMedGoogle Scholar
- Luchavez J, Espino F, Curameng P, Espina R, Bell D, Chiodini P, Human infections with Plasmodium knowlesi, the Philippines. Emerg Infect Dis. 2008;14:811–3. DOIPubMedGoogle Scholar
- Berens-Rihas N. Plasmodium knowlesi found in several samples from Indonesia. ProMed. 2009 Jun 21 [cited 2010 Jan 12]. http://www.promedmail.org, archive no. 20090621.2278.
- Lee KS, Cox-Singh J, Singh B. Morphological features and differential counts of Plasmodium knowlesi parasites in naturally acquired human infections. Malar J. 2009;8:73. DOIPubMedGoogle Scholar
- McCutchan TF, Piper RC, Makler MT. Use of malaria rapid diagnostic test to identify Plasmodium knowlesi infection. Emerg Infect Dis. 2008;14:1750–2.PubMedGoogle Scholar
- Knowles RM, DasGupta BM. A study of monkey-malaria and its experimental transmission to man. Ind Med Gaz. 1932;67:301–20.
- Cox-Singh J, Singh B. Knowlesi malaria: newly emergent and of public health importance? Trends Parasitol. 2008;24:406–10. DOIPubMedGoogle Scholar
- Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans R Soc Trop Med Hyg. 2006;100:1087–8. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 16, Number 4—April 2010
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Melanie Figtree, Department of Microbiology and Infectious Diseases, Royal North Shore Hospital, Pacific Hwy, St. Leonards, Sydney, New South Wales 2065, Australia
Top