Volume 19, Number 6—June 2013
CME ACTIVITY - Research
Foodborne Botulism in Canada, 1985–2005
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: May 22, 2013; Expiration date: May 22, 2014
Learning Objectives
Upon completion of this activity, participants will be able to:
• Describe the incidence of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada, based on a study of laboratory databases.
• Describe the implicated pathogens and sources of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada, based on a study of laboratory databases.
• Describe the outcomes of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada, based on a study of laboratory databases.
CME Editor
Shannon O’Connor, ELS, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Shannon O’Connor has disclosed no relevant financial relationships.
CME Author
Laurie Barclay, MD, freelance writer and reviewer, Medscape, LLC. Disclosure: Laurie Barclay, MD, has disclosed no relevant financial relationships.
Authors
Disclosures: Daniel Leclair, PhD,; Joe Fung, MSc, MPH; Judith L. Isaac-Renton, MD; Jean-Francois Proulx, MD; Jennifer May-Hadford, MPH; Andrea Ellis; Edie Ashton; Sadjia Bekal, PhD; Jeffrey M. Farber, PhD; Burke Blanchfield; and John W. Austin, PhD, have disclosed no relevant financial relationships.
During 1985–2005, a total of 91 laboratory-confirmed outbreaks of foodborne botulism occurred in Canada; these outbreaks involved 205 cases and 11 deaths. Of the outbreaks, 75 (86.2%) were caused by Clostridium botulinum type E, followed by types A (7, 8.1%) and B (5, 5.7%). Approximately 85% of the outbreaks occurred in Alaska Native communities, particularly the Inuit of Nunavik in northern Quebec and the First Nations population of the Pacific coast of British Columbia. These populations were predominantly exposed to type E botulinum toxin through the consumption of traditionally prepared marine mammal and fish products. Two botulism outbreaks were attributed to commercial ready-to-eat meat products and 3 to foods served in restaurants; several cases were attributed to non-Native home-prepared foods. Three affected pregnant women delivered healthy infants. Improvements in botulism case identification and early treatment have resulted in a reduction in the case-fatality rate in Canada.
Foodborne botulism, a notifiable disease in Canada, results from the ingestion of foods contaminated with preformed botulinum neurotoxin types A, B, E, or F, produced by Clostridium botulinum groups I and II (1). More rarely, outbreaks of foodborne botulism in the United States, India, and China have been caused by neurotoxigenic C. butyricum type E (2,3) and C. baratii type F (4). In Canada, C. botulinum type E has been the most common serotype since the first type E outbreak in 1944 in Nanaimo, British Columbia (reported in 1947) (5–7).
Six forms of botulism have been described in the literature (8), but only foodborne and infant botulism and rare cases of adult colonization have been reported in Canada (1,9). Regardless of the form or serotypes involved, however, human botulism is a medical emergency that requires rapid intervention. Because prompt administration of antitoxin can reduce the severity of the disease (10), the decision for treatment is based on clinical diagnosis and epidemiologic information, without laboratory confirmation.
Investigation of foodborne botulism incidents provides useful information regarding implicated foods and conditions resulting in toxin formation. The last epidemiologic review on foodborne botulism in Canada was done for the 1971–1984 period (7). Since then, annual summaries of botulism cases were inconsistently published through disease surveillance reports (e.g., 11,12). Here, we summarize reports of all laboratory-confirmed cases of foodborne botulism in Canada during 1985–2005.
Data Sources
Two independent laboratory databases, maintained by the Botulism Reference Service at Health Canada, Ottawa, Ontario, and the British Columbia Public Health Reference Microbiology Laboratory, Vancouver, British Columbia, were examined for cases of foodborne botulism confirmed during 1985–2005. Information regarding the number of clinical cases, age and sex of patients, implicated food, case history, laboratory analysis, date, and location of the outbreak were extracted.
To ensure consistency in data recording and analysis throughout the study period, we used the 2009 national case definition for confirmed cases of foodborne botulism (13). A case of foodborne botulism is confirmed on the basis of clinical evidence and a positive laboratory specimen (i.e., detection of botulinum neurotoxin in serum, feces, gastric aspirate, or food or isolation of C. botulinum from feces or gastric aspirate). In addition to cases with laboratory confirmation, persons with botulism who were epidemiologically linked to a laboratory-confirmed case were considered to have confirmed, and thus reportable, cases. Detection of botulinum neurotoxin and isolation of viable C. botulinum from foods and clinical specimens were performed according to Health Canada method MFHPB-16 (14). Data on length of hospitalization were retrieved from the Hospital Morbidity Database (HMDB) of the Canadian Institute for Health Information (www.cihi.ca); all records that listed botulism in the first 3 suspected diagnostic codes were extracted for the years 1994–2005 (all years currently available). These records were then matched to the laboratory records by age, sex, date of admission, date of sample, and province of residence. Only cases with laboratory confirmation were included in the analysis of the HMDB data.
Data Analysis
To provide a descriptive epidemiology of outbreaks of foodborne botulism in Canada during 1985–2005, we analyzed data from laboratory-confirmed outbreaks (in Canada, because of the urgency of the disease, 1 case of botulism constitutes an outbreak) in terms of case numbers and rates, demographic characteristics of patients, length of hospitalization, food types, outbreak settings, serotype, and circumstances of occurrence. The rates of disease were calculated by using census data from Statistics Canada and other sources (15,16). Statistical analysis of the HMDB data was done in SPSS version 19 (IBM, Armonk, NY, USA). The Mann-Whitney test was done to compare central tendency of the data; relationships were considered significant at p<0.05.
Demographics and Incidence
Of 91 laboratory-confirmed outbreaks (a total of 205 cases), C. botulinum type E was implicated in 75 (86.2%), followed by types A (7, 8.1%) and B (5, 5.7%). Median patient age was 45 years (range 3–80 years); 93 (48.4%) were male. Three cases in the Nunavik region were recorded as repeated episodes of type E botulism, with the second episodes occurring 10–20 years after the initial intoxication. Overall, the number of outbreaks of foodborne botulism did not decrease during the study period, with a mean of 4.3 outbreaks/year. Outbreaks involved 1–37 cases, and 78% of outbreaks involved 1 or 2 cases. Mean annual incidence was 0.03 cases/100,000 population. The annual number of cases was marked by peaks associated with large outbreaks (Figure 1). The number of cases appeared to decrease during the last 5 years of the study period; lower numbers of outbreaks with multiple cases were reported (Table 1).
Morbidity and Mortality
A total of 11 deaths were reported (case-fatality rate 5.4%). Within the 21-year study period, the case-fatality rate declined from 8.8% in 1985–1989 to 2.4% in 1990–1994 and then remained below 3.6% during the remaining 5-year intervals (Table 1). However, because mortality data were derived from the laboratory databases that do not officially keep mortality records, these data should be interpreted with caution.
Three of the reported cases occurred in pregnant women. Case history revealed that 2 of these women were exposed to type E botulinum neurotoxin and 1 to type B botulinum neurotoxin. No apparent effect on pregnancy was reported.
Severity of illness was assessed by using HMDB data for 1994–2005. The length of hospitalization was obtained for 70 patients with type E botulism (Table 2); the low number of type A and type B botulism cases recorded in HMDB did not enable valid data reporting. Although HMDB does not list deaths, the authors are aware of 1 fatal type E case from among these patients. Of botulism type E case-patients with known length of hospitalization, 37 (52.9%) were female and 33 male, which was not significantly different (p = 0.633). The median length of hospitalization was 7 days for female (mean 14.8 days) and male (mean 8.7 days) patients; these findings were not significantly different (p>0.05). The mean for the female group was influenced by 1 fatal case in which the patient was hospitalized for 225 days. When type E case-patients were subdivided based on age, age groups differed in length of hospitalization, but a trend was not apparent (Table 2).
Hospital procedure codes were extracted from HMDB for 54 patients with type E botulism. These 54 cases represent only a subset of the total type E cases, and all were laboratory confirmed and matched with laboratory records. Intubation alone was recorded for 16 (30%) patients, antitoxin alone for 21 (39%) patients, and both for 5 (9%) patients; 12 (22%) patients received neither antitoxin nor ventilation.
For the group comprising all patients that received antitoxin (n = 26), the median length of hospital stay was 5 days (mean 15.1 days, SD 43.2), significantly (p = 0.003) shorter than for the group that did not receive antitoxin (n = 28), which had a median length of hospital stay of 11 days (mean 13.3 days, SD 8.32). For the group comprising all patients that were intubated (n = 21), the median length of hospital stay was 13 days (mean 24.5 days, SD 46.6), significantly (p<0.001) longer than for the group that did not undergo intubation (n = 33), which had a median length of hospital stay of 5 days (mean 7.58 days, SD 6.24). Intubation is likely a marker of severity of symptoms and is not thought to be the cause of increased length of hospitalization. No determination for the elapsed time period between symptom onset and the administration of antitoxin or ventilation was available.
Geographic Distribution
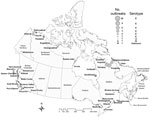
Most (85, 93.4%) confirmed botulism outbreaks originated in Quebec, British Columbia, Nunavut, and the Northwest Territories. Reported cases predominantly occurred in Quebec and British Columbia, with 89 and 71 cases, respectively; these cases accounted for 78% of the total number of cases (Table 3). Of 51 outbreaks in Quebec, 45 (88%) occurred in the Nunavik region of northern Quebec; 91% of these were clustered in 3 villages of southern Ungava Bay (Kuujjuaq, Kangiqsualujjuaq, and Tasiujaq), which are inhabited by an Inuit population of 2,587 (Figure 2). In British Columbia, 9 (64%) of 14 outbreaks (20 cases) occurred in First Nations communities located along the Pacific Coast. The high number of cases recorded in the province was primarily because of 2 large restaurant-associated outbreaks in Vancouver that affected 37 persons (17) and 11 persons (18). In Ontario, 3 outbreaks were recorded, with 1 affecting 3 persons and causing 1 death. No botulism cases were reported in the provinces of Alberta, Saskatchewan, Manitoba, New Brunswick, Nova Scotia, and Prince Edward Island during the study period.
Traditional Native Foods
Other than a single outbreak in Nunavut caused by consumption of fermented fish heads, all type E outbreaks with known food sources in northern Canada were linked to marine mammals, including beluga whales, seals, and walruses (Table 4). Of the 41 botulism outbreaks in Nunavik, 32 (78%) were caused by food products of seal origin; aged meat (igunaq) and aged flippers (utjaq) were most frequently implicated foods in Nunavik, accounting for 15 (47%) seal-related outbreaks. Beluga whale was involved in 16 (80%) of 20 outbreaks in Nunavut and the Northwest Territories. Eleven of these outbreaks were caused by muktuk, which is aged pieces of skin (with fat and meat) of the beluga whale.
In the First Nations communities of British Columbia, 9 outbreaks of type E botulism were associated with the consumption of aged fish products. Fermented salmon eggs (stink eggs) have been the primary source of botulism, accounting for 8 of 9 (89%) outbreaks in British Columbia coastal communities. Because no carbohydrates are available in these eggs for a fermentative transformation into organic acids, the aging process involves putrefaction rather than fermentation.
Restaurant Food
The 1985 type B outbreak involving garlic-in-oil at a Vancouver restaurant affected 37 persons; 24 were hospitalized, and none died (17) (Table 4). The outbreak occurred in 2 clusters, with 11 cases in the first cluster during July 28–August 2, 1985, and 26 cases in the second cluster during August 29–September 5, 1985. In this incident, garlic-in-oil used in sandwiches was kept at room temperature for 8 months. In a 1987 botulism outbreak at a hotel restaurant in Vancouver, 11 persons became ill after consuming bottled chanterelle mushrooms contaminated with type A botulinum neurotoxin (18). The mushrooms were grown on Vancouver Island and bottled at the hotel using an in-house heating process. One jar recovered during the investigation was found to contain 4,000 minimal lethal dose/mL of type A neurotoxin in the liquid phase.
A third outbreak associated with a restaurant occurred in Ontario in 2002 and involved a single case of type A botulism linked to a baked potato (19). This patient began to show gastro-intestinal and neurologic symptoms 12 hours after eating the potato, the remains of which had been discarded and was unavailable for testing. The patient was hospitalized for >6 months and released with long-term sequelae; the patient continued to experience weakness and vision problems 4 years after the incident.
Commercial Foods
Two unrelated incidents involving commercial ready-to-eat meat products were reported in Quebec in 1995 and 2001 (Table 4). In both instances, products that were intended to be refrigerated were stored at room temperature, which enabled growth of C. botulinum and toxin production. In the 1995 incident, C. botulinum type B was isolated from fecal samples of 2 persons and from a commercial country-style pâté. In the 2001 incident, C. botulinum type A was isolated from a cooked boneless pork product and from fecal and gastric liquid samples from a patient. The cooked boneless pork product was recalled as a precautionary measure.
Home-prepared Foods
Several incidents during the 21-year study period involved non-Native home-prepared foods (Table 4). Home-canned mushrooms were linked to a type B botulism incident that affected 1 person in Quebec. Home-canned asparagus was involved in 1 type A botulism incident in Ontario; 3 persons required intensive care, and 1 died. In this incident, 8 of 10 glass jars of whole-stalk asparagus showed evidence of odorous gas production during the investigation. One additional type A case was linked to the consumption of home-canned vegetable and beef soup.
A variety of noncanned home-prepared foods, including bean soup, spaghetti sauce, sausages, and fish, were also implicated in botulism outbreaks, although most of the incidents could not be laboratory confirmed because no remaining food was available. Home-smoked fish was responsible for 3 cases of type E botulism, with 1 fatality, in the Yukon in 1995. Diagnosis and treatment with antitoxin was delayed until 3 days after the onset of symptoms. Another incident of fish consumption affected 1 tourist traveling in Quebec in 2001, but the mode of preparation was not recorded. Home-prepared spaghetti sauce made with home-canned sausage caused botulism in 1 person from Quebec in 2000. Another type A case was associated with the consumption of homemade bean soup, but its preparation was not documented.
Laboratory Findings
Of 91 total confirmed outbreaks in Canada during the study period, 77 (84.6%) were confirmed by detection of botulinum neurotoxin only or by detection of neurotoxin and isolation of C. botulinum from laboratory specimens. The remaining 14 outbreaks were confirmed by detection of C. botulinum in gastric contents and/or fecal samples only, without detection of neurotoxin in clinical samples. Food was recovered in 69 (75.8%) of the outbreak investigations. The detection rate for botulinum neurotoxin in foods (78.3%) was found to be higher than for serum (34.9%), feces (34.5%), or gastric contents (11.0%) (Table 5). C. botulinum was also more frequently detected in foods (83.3%), followed by gastric contents (51.3%) and feces (40.3%). Two botulism cases were confirmed by the detection of C. botulinum in liver tissue during autopsies.
The annual mean of 4.3 confirmed botulism outbreaks during 1985–2005 was identical to that reported for the 1971–1984 period (7); however, the case-fatality rate dramatically decreased during 1985–2005, from 17% to 5.4%. This decrease could reflect the increased awareness of symptoms in high-risk communities, which leads to prompt medical attention and the administration of antitoxin. Similar to 1971–1984, most cases occurred in Quebec, British Columbia, Nunavut, and the Northwest Territories, which is likely a reflection of the rate of consumption of higher-risk traditional foods in these provinces and territories. The annual mean number of outbreaks for British Columbia decreased from 0.9 to 0.7, whereas the rate for Quebec remained unchanged at 2.4 outbreaks per year. The Northwest Territories, Nunavut, and Yukon combined had 21 outbreaks during the study period, an annual average of 1 outbreak per year, unchanged from 1971–84 (7).
Botulism remains a public health challenge in many communities where Native foods are persistently incriminated (7). The mean incidence rate for all of Canada is low and is similar to that of the United States (20), but the rate among the Native population of Nunavik, where type E botulism linked to aged marine mammal products is endemic, is >1,600 times higher than for the rest of Canada (0.03/100,000 population for Canada vs. 50.5/100,000 population for Nunavik). Outbreaks of botulism associated with Native foods in Canada account for 83.5% of all outbreaks; in the United States, 36.3% of all outbreaks were associated with Native foods in Alaska (20).
Previous epidemiologic studies have identified the food types and the modes of preservation of foods frequently associated with type E botulism in northern Canada (6,7). Aged marine mammals and fish were the predominant vehicles of type E botulism before 1985 and remain responsible for most type E botulism cases in Canada. The risk for contamination of marine mammal meat during the butchering process performed under field conditions is high because of the ubiquitous presence of the organism in the coastal environment (21,22). Measures to minimize field contamination of marine mammal tissues with type E spores during the butchering and preparation of meat, fat, or skin have recently been developed (23). In collaboration with regional health authorities, studies directed at controlling the growth of C. botulinum type E in aged marine mammal products have indicated that storage temperatures <3°C will prevent toxin production during the aging process of Native foods (23,24).
Food service establishments are potential risk settings where widespread public exposure to contaminated foods may occur. The garlic-in-oil incident in 1985 was the first reported Canadian botulism outbreak associated with a food service establishment and was the largest ever reported botulism outbreak in Canada. This incident, along with a similar incident that occurred in the United States in 1989, led to regulatory changes requiring inclusion of acidifying agents in commercial garlic-in-oil products (25). The restaurant outbreak involving bottled chanterelle mushrooms in 1987 led the Canadian Restaurant and Foodservices Association to modify its sanitation code to recommend that in-house canned or bottled foods not be served in restaurants.
The number of botulism incidents associated with foods sold at retail remained low, with only 2 outbreaks causing illness to 3 persons. Non-Native home-prepared foods were implicated in 8 outbreaks in Canada; 2 of these were caused by home-canned vegetables. Continuous education is needed to inform consumers of the potential risks of botulism from eating home-prepared foods and to promote the use of a pressure canner according to manufacturer’s instructions and proper food storage temperatures in the home.
In addition to the classic diagnostic samples (i.e., serum, gastric content, feces, and suspect foods), the confirmation of 2 cases in 1985 was completed with the detection of viable C. botulinum in liver tissue during postmortem examination. Detection of viable C. botulinum in postmortem liver samples has been reported previously (7,26).
We found that 3 pregnant women who were diagnosed with botulism had no reported pregnancy complications. One of the patients had persistent toxemia for >10 days during the first trimester of pregnancy and later delivered a normal infant. Five previous cases of botulism during pregnancy have been reported; none of the infants appeared to have clinical botulism at birth (27–31). No evidence shows that botulinum toxin can cross the placenta (32) or that the neuromuscular effects of botulinum toxin on the mother would affect the development of the fetus (28).
Type A botulism is recognized to be more severe than type E botulism because of the higher proportion of patients who require intubation (33). Hughes et al. reported the mean length of hospitalization as 63 days for type A and 21 days for type B botulism (34), longer than the 11.9 days determined in this study for type E botulism. Administration of antitoxin has been shown to reduce risk for death and shorten the course of type A botulism (10). The data in this study showed that antitoxin shortened the median length of hospitalization for patients with type E botulism from 11 days to 5 days.
The overall case-fatality rate from botulism in Canada has decreased from 17.2% to 5.4%, approaching the rate in United States (20), which can likely be attributed to the early recognition and medical management of type E botulism cases in First Nations and Inuit communities. Most foodborne botulism outbreaks in Canada continue to occur among First Nations and Inuit people, a trend that has not changed since a 1974 comprehensive review of botulism cases, which attributed most cases of type E botulism to aged marine mammal and fish products (6). However, extensive progress has been made in improving case identification and early treatment, which has led to a substantial decrease in the case-fatality rate.
Dr Leclair is a veterinarian and chief of Food Microbiology Surveys at the Canadian Food Inspection Agency. At the time of this research, he was a PhD student in the Biochemistry, Microbiology and Immunology Department of the University of Ottawa. His research interests include the epidemiology and prevention of foodborne diseases in Arctic regions.
Acknowledgment
We thank Greg Sanders for technical assistance and Bruce Craig for mapping botulism cases.
References
- Austin JW. Clostridium botulinum. In: Doyle MP, Beuchat LR, Montville TJ, editors. Food microbiology. Fundamentals and frontiers. Washington (DC): ASM Press; 2001. p. 329–49.
- Chaudhry R, Dhawan B, Kumar D, Bhatia R, Gandhi JC, Patel RK, Outbreak of suspected Clostridium butyricum botulism in India. Emerg Infect Dis. 1998;4:506–7. DOIPubMedGoogle Scholar
- Meng X, Karasawa T, Zou K, Kuang X, Wang X, Lu C, Characterization of a neurotoxigenic Clostridium butyricum strain isolated from the food implicated in an outbreak of food-borne type E botulism. J Clin Microbiol. 1997;35:2160–2 .PubMedGoogle Scholar
- Harvey SM, Sturgeon J, Dassey DE. Botulism due to Clostridium baratii type F toxin. J Clin Microbiol. 2002;40:2260–2. DOIPubMedGoogle Scholar
- Dolman CE, Kerr DE. Botulism in Canada, with report of a type E outbreak at Nanaimo, B.C. Can J Public Health. 1947;38:48–57 .PubMedGoogle Scholar
- Dolman CE. Human botulism in Canada (1919–1973). Can Med Assoc J. 1974;110:191–200 .PubMedGoogle Scholar
- Hauschild AH, Gauvreau L. Food-borne botulism in Canada, 1971–84. CMAJ. 1985;133:1141–6 .PubMedGoogle Scholar
- Sheppard YD, Middleton D, Whitfield Y, Tyndel F, Haider S, Spiegelman J, Intestinal toxemia botulism in 3 adults, Ontario, Canada, 2006–2008. Emerg Infect Dis. 2012;18:1–6. DOIPubMedGoogle Scholar
- Tacket CO, Shandera WX, Mann JM, Hargrett NT, Blake PA. Equine antitoxin use and other factors that predict outcome in type A foodborne botulism. Am J Med. 1984;76:794–8. DOIPubMedGoogle Scholar
- Hauschild A, Gauvreau L, Black WA. Botulism in Canada—summary for 1985. Can Dis Wkly Rep. 1986;12:53–4.
- Austin J, Blanchfield B, Ashton E, Lorange M, Proulx JF, Trinidad A, Botulism in Canada—summary for 1997. Can Commun Dis Rep. 1999;25:121–2 .PubMedGoogle Scholar
- Case definitions for diseases under national surveillance. Can Commun Dis Rep. 2009;2009:35.
- Austin JW, Sanders G. Detection of Clostridium botulinum and its toxins in suspect foods and clinical specimens. Compendium of analytical methods. 2009 [cited 2013 Apr 4]. http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/mfhpb16-eng.pdf
- Aboriginal peoples in Canada in 2006: Inuit, Métis and First Nations, 2006 census. Ottawa (Ontario, Canada): Statistics Canada; 2008.
- Nunivaat. Population of beneficiaries by age and sex, 1978 to 2005. Université Laval (QC); 2007.
- St Louis ME, Peck SH, Bowering D, Morgan GB, Blatherwick J, Banerjee S, Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med. 1988;108:363–8 . DOIPubMedGoogle Scholar
- Centers for Disease Control. Restaurant-associated botulism from mushrooms bottled in-house—Vancouver, British Columbia, Canada. MMWR Morb Mortal Wkly Rep. 1987;36:103 .PubMedGoogle Scholar
- Bhutani M, Ralph E, Sharpe MD. Acute paralysis following “a bad potato”: a case of botulism. Can J Anaesth. 2005;52:433–6. DOIPubMedGoogle Scholar
- Sobel J, Tucker N, Sulka A, McLaughlin J, Maslanka S. Foodborne botulism in the United States, 1990–2000. Emerg Infect Dis. 2004;10:1606–11. DOIPubMedGoogle Scholar
- Miller LG. Observations on the distribution and ecology of Clostridium botulinum type E in Alaska. Can J Microbiol. 1975;21:920–6 and. DOIPubMedGoogle Scholar
- Leclair D, Farber JM, Doidge B, Blanchfield B, Suppa S, Pagotto F, Distribution of Clostridium botulinum type E in Nunavik, Northern Quebec. Appl Environ Microbiol. 2013;79:646–54. DOIPubMedGoogle Scholar
- Leclair D. Molecular epidemiology and risk assessment of human botulism in the Canadian Arctic. Ottawa (Ontario, Canada): University of Ottawa; 2008.
- Austin JW, Leclair D. Botulism in the North: a disease without borders. Clin Infect Dis. 2011;52:593–4. DOIPubMedGoogle Scholar
- Morse DL, Pickard LK, Guzewich JJ, Devine BD, Shayegani M. Garlic-in-oil associated botulism: episode leads to product modification. Am J Public Health. 1990;80:1372–3. DOIPubMedGoogle Scholar
- Dubovsky BJ, Meyer KF. An experimental study of the methods available for the enrichment, demonstration and isolation of B. botulinus in specimens of soil and its products, in suspected food, in clinical and necropsy material. I. J Infect Dis. 1922;31:501–40. DOIGoogle Scholar
- Morrison GA, Lang C, Huda S. Botulism in a pregnant intravenous drug abuser. Anaesthesia. 2006;61:57–60. DOIPubMedGoogle Scholar
- Robin L, Herman D, Redett R. Botulism in a pregnant woman. N Engl J Med. 1996;335:823–4. DOIPubMedGoogle Scholar
- Magri K, Bresson V, Barbier C. Botulisme et grossesse. J Gynecol Obstet Biol Reprod (Paris). 2006;35:624–6. DOIPubMedGoogle Scholar
- Polo JM, Martin J, Berciano J. Botulism and pregnancy. Lancet. 1996;348:195. DOIPubMedGoogle Scholar
- St Clair EH, DiLiberti JH, O’Brien ML. Observations of an infant born to a mother with botulism. J Pediatr. 1975;87:658. DOIPubMedGoogle Scholar
- Aranda MA, Herranz A, del Val J, Bellido S, García-Ruiz P. Botulinum toxin A during pregnancy, still a debate. Eur J Neurol. 2012;19:e81–2. DOIPubMedGoogle Scholar
- Woodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975–1988. J Infect Dis. 1992;166:1281–6 . DOIPubMedGoogle Scholar
- Hughes JM, Blumenthal JR, Merson MH. Clinical features of types A and B food-borne botulism. Ann Intern Med. 1981;95:442–5 . DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 70% passing score) and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title:
Foodborne Botulism in Canada, 1985–2005
CME Questions
1. You are an infectious disease consultant to a public health department in Canada regarding the likelihood of botulism outbreaks. Based on the laboratory database study by Dr. Leclair and colleagues, which of the following statements about the incidence of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada is most likely to appear in your report?
A. There were 91 laboratory-confirmed outbreaks of foodborne botulism, involving 205 cases
B. The annual rate of confirmed outbreaks throughout Canada was significantly lower than that reported for 1971–1984
C. About half of the outbreaks occurred in native communities
D. Outbreaks are less likely among the Nunavut than in Canada as a whole
2. Based on the laboratory database study by Dr. Leclair and colleagues, which of the following statements about pathogens and sources of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada is most likely to appear in your report?
A. Clostridium botulinum type B was the most common pathogen
B. Commercial ready-to-eat meat products were the most common source
C. None of the outbreaks involved food served in restaurants
D. Continuous consumer education is needed on the potential risks of botulism from home-prepared foods, use of a pressure canner, and proper food storage temperatures
3. Based on the laboratory database study by Dr. Leclair and colleagues, which of the following statements about outcomes of laboratory-confirmed outbreaks of foodborne botulism occurring between 1985 and 2005 in Canada would most likely be correct?
A. No change in case identification from the period before 1985 resulted in no change in the case fatality rate
B. Of the 205 reported cases, 11 died
C. Of 3 cases in pregnant women, 1 resulted in stillbirth
D. Antitoxin administration was not associated with reduction in hospital length of stay in type E cases
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
Related Links
Table of Contents – Volume 19, Number 6—June 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
John W. Austin, Health Canada Microbiology, 251 Sir Frederick Banting Driveway, Ottawa, ON K1A 0K9, Canada
Top