Volume 20, Number 1—January 2014
Research
Multisite Validation of Cryptococcal Antigen Lateral Flow Assay and Quantification by Laser Thermal Contrast
Figure 3
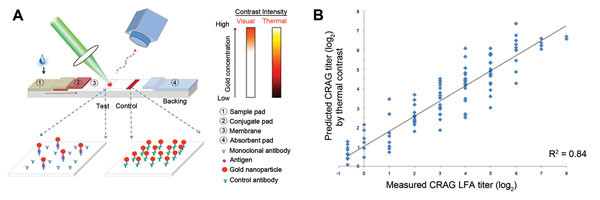
Figure 3. . A) Prediction of cryptococcal antigen titer based on laser thermal contrast measurement and concept of lateral flow immunochromatographic assay (LFA) thermal contrast measurement in which a laser irradiates the test line in the LFA (19). The test line is formed by gold–monoclonal antibody–antigen sandwich complex with a monoclonal antibody affixed at the test line. When irradiated by a green laser (532 nm), any gold present absorbs light from the laser and generates heat in direct proportion to the amount of gold (and thereby antigen) present at the test line. This temperature change can be measured by using an infrared camera. B) Association of measured semiquantitative LFA cryptococcal antigen (CRAG) titer starting at a 1:250 dilution by the predicted CRAG titer based on thermal contrast measurement. Measurements on the negative portion of the x-axis are beyond the visual range when specimens were diluted 1:250, yet still detectable by thermal contrast. The Pearson correlation coefficient was r = 0.91 (p<0.001, R2 = 0.84) among 115 positive specimens quantified. A total of 58 LFA CRAG–negative specimens established background levels of heat radiation.
References
- Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infect Dis. 2010;10:67 .DOIPubMedGoogle Scholar
- Hakim JG, Gangaidzo IT, Heyderman RS, Mielke J, Mushangi E, Taziwa A, Impact of HIV infection on meningitis in Harare, Zimbabwe: a prospective study of 406 predominantly adult patients. AIDS. 2000;14:1401–7 .DOIPubMedGoogle Scholar
- Cohen DB, Zijlstra EE, Mukaka M, Reiss M, Kamphambale S, Scholing M, Diagnosis of cryptococcal and tuberculous meningitis in a resource-limited African setting. Trop Med Int Health. 2010;15:910–7 .DOIPubMedGoogle Scholar
- Durski KN, Kuntz KM, Yasukawa K, Virnig BA, Meya DB, Boulware DR. Cost-effective diagnostic checklists for meningitis in resource-limited settings. J Acquir Immune Defic Syndr. 2013;63:e101–8 .DOIPubMedGoogle Scholar
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30 .DOIPubMedGoogle Scholar
- French N, Gray K, Watera C, Nakiyingi J, Lugada E, Moore M, Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–8 .DOIPubMedGoogle Scholar
- Liechty CA, Solberg P, Were W, Ekwaru JP, Ransom RL, Weidle PJ, Asymptomatic serum cryptococcal antigenemia and early mortality during antiretroviral therapy in rural Uganda. Trop Med Int Health. 2007;12:929–35 .DOIPubMedGoogle Scholar
- World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children, 2011 [cited 2012 May 31]. http:// www.who.int/hiv/pub/cryptococcal_disease2011
- Jarvis JN, Govender N, Chiller T, Park BJ, Longley N, Meintjes G, Cryptococcal antigen screening and preemptive therapy in patients initiating antiretroviral therapy in resource-limited settings: a proposed algorithm for clinical implementation. J Int Assoc Physicians AIDS Care (Chic). 2012;11:374–9 .DOIPubMedGoogle Scholar
- Rajasingham R, Meya DB, Boulware DR. Integrating cryptococcal antigen screening and pre-emptive treatment into routine HIV care. J Acquir Immune Defic Syndr. 2012;59:e85–91 .DOIPubMedGoogle Scholar
- Bicanic T, Harrison TS. Cryptococcal meningitis. Br Med Bull. 2004;72:99–118 .DOIPubMedGoogle Scholar
- Kambugu A, Meya DB, Rhein J, O’Brien M, Janoff EN, Ronald AR, Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46:1694–701 .DOIPubMedGoogle Scholar
- Kisenge PR, Hawkins AT, Maro VP, McHele JP, Swai NS, Mueller A, Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis. 2007;7:39 .DOIPubMedGoogle Scholar
- Trachtenberg JD, Kambugu AD, McKellar M, Semitala F, Mayanja-Kizza H, Samore MH, The medical management of central nervous system infections in Uganda and the potential impact of an algorithm-based approach to improve outcomes. Int J Infect Dis. 2007;11:524–30 .DOIPubMedGoogle Scholar
- Brouwer AE, Teparrukkul P, Pinpraphaporn S, Larsen RA, Chierakul W, Peacock S, Baseline correlation and comparative kinetics of cerebrospinal fluid colony-forming unit counts and antigen titers in cryptococcal meningitis. J Infect Dis. 2005;192:681–4 .DOIPubMedGoogle Scholar
- Taseera K, Siedner MJ, Klausner JD, Muzoora C, Boulware DR. Point-of-care diagnosis and prognostication of cryptococcal meningitis with the cryptococcal antigen lateral flow assay on cerebrospinal fluid. [Epub ahead of print]. Clin Infect Dis. 2013.
- Diamond RD, Bennett JE. Prognostic factors in cryptococcal meningitis. A study in 111 cases. Ann Intern Med. 1974;80:176–81 .DOIPubMedGoogle Scholar
- Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384 .DOIPubMedGoogle Scholar
- Qin Z, Chan WC, Boulware DR, Akkin T, Butler EK, Bischof JC. Significantly improved analytical sensitivity of lateral flow immunoassays by using thermal contrast. Angew Chem Int Ed Engl. 2012;51:4358–61 .DOIPubMedGoogle Scholar
- Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A, Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962–70 .DOIPubMedGoogle Scholar
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet. 2004;363:1764–7 .DOIPubMedGoogle Scholar
- Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral-naive or antiretroviral-experienced patients treated with amphotericin B or fluconazole. Clin Infect Dis. 2007;45:76–80 .DOIPubMedGoogle Scholar
- Wiesner DL, Moskalenko O, Corcoran JM, McDonald T, Rolfes MA, Meya DB, Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3:e00196–12 .DOIPubMedGoogle Scholar
- Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN, Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011;53:1019–23 .DOIPubMedGoogle Scholar
- Gates-Hollingsworth MA, Kozel TR. Serotype sensitivity of a lateral flow immunoassay for cryptococcal antigen. Clin Vaccine Immunol. 2013;20:634–5 .DOIPubMedGoogle Scholar
- Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess. 2007;11:iii: ix–51.
- Meya DB, Manabe YC, Castelnuovo B, Cook BA, Elbireer AM, Kambugu A, Cost-effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count ≤100 cells/microL who start HIV therapy in resource-limited settings. Clin Infect Dis. 2010;51:448–55 .DOIPubMedGoogle Scholar
- Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis. 2009;48:856–62 .DOIPubMedGoogle Scholar
- Joint Medical Store. Product catalogue. 2013 June 5, [cited 2013 Sep 1]. http://www.jms.co.ug/resources
- Lindsley MD, Mekha N, Baggett HC, Surinthong Y, Autthateinchai R, Sawatwong P, Evaluation of a newly developed lateral flow immunoassay for the diagnosis of cryptococcosis. Clin Infect Dis. 2011;53:321–5 .DOIPubMedGoogle Scholar
- Binnicker MJ, Jespersen DJ, Bestrom JE, Rollins LO. Comparison of four assays for the detection of cryptococcal antigen. Clin Vaccine Immunol. 2012;19:1988–90 .DOIPubMedGoogle Scholar
- Hansen J, Slechta ES, Gates-Hollingsworth MA, Neary B, Barker AP, Bauman S, Large-scale evaluation of the immuno-mycologics lateral flow and enzyme-linked immunoassays for detection of cryptococcal antigen in serum and cerebrospinal fluid. Clin Vaccine Immunol. 2013;20:52–5 .DOIPubMedGoogle Scholar
- McMullan BJ, Halliday C, Sorrell TC, Judd D, Sleiman S, Marriott D, Clinical utility of the cryptococcal antigen lateral flow assay in a diagnostic mycology laboratory. PLoS ONE. 2012;7:e49541 .DOIPubMedGoogle Scholar
- Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207 .PubMedGoogle Scholar
- Govender NP, Meintjes G, Bicanic T, Dawood H, Harrison TS, Jarvis JN, Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. South African Journal of HIV Medicine. 2013;14:76–86 .DOIGoogle Scholar
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291–322 .DOIPubMedGoogle Scholar
- Lortholary O, Poizat G, Zeller V, Neuville S, Boibieux A, Alvarez M, Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–91 .DOIPubMedGoogle Scholar
- Sungkanuparph S, Filler SG, Chetchotisakd P, Pappas PG, Nolen TL, Manosuthi W, Cryptococcal immune reconstitution inflammatory syndrome after antiretroviral therapy in AIDS patients with cryptococcal meningitis: a prospective multicenter study. Clin Infect Dis. 2009;49:931–4 .DOIPubMedGoogle Scholar