Volume 21, Number 8—August 2015
Dispatch
Ribavirin for Chronic Hepatitis Prevention among Patients with Hematologic Malignancies
Abstract
Findings among a cohort of 26 patients who had hematologic malignancies and hepatitis E virus (HEV) infection support that HEV can induce chronic hepatitis. However, a 3-month course of ribavirin can induce a rapid viral clearance, reducing the risk for chronic hepatitis and enabling continuation of cytotoxic treatments for underlying malignancies.
Hepatitis E virus (HEV) is a nonenveloped RNA virus transmitted by consumption of contaminated water, undercooked infected pork or wild boar, deer, or rabbit meats (1), or by blood transfusion or solid organ transplant (SOT) (2). In addition to acute hepatitis, HEV causes chronic hepatitis and cirrhosis in immunocompromised patients (3,4). Knowledge of hepatitis E in patients with hematologic cancers is mostly derived from case reports or small series (5–10). We previously described 6 patients who had various hematological malignancies and HEV infection and reported chronic hepatitis in 3 of them (6). Management of HEV infection in the context of allogeneic stem cell transplantation (SCT) has been a matter of controversy (5,6). Ribavirin is the treatment of choice in patients infected during SOT (11). In hematologic patients infected with HEV, data concerning efficacy and safety of ribavirin are scarce (12).
During 2003–2009, patients at Institut Universitaire du Cancer, Toulouse, were randomly tested for HEV infection; patients who had elevated liver enzymes were routinely assessed for the virus. Patients were generally counseled to avoid undercooked foods or products that increased risk for HEV transmission according to guidelines from the French Ministry of Health. All patients with a diagnosis of HEV infection were counseled. HEV RNA was detected and quantified by PCR testing, and HEV IgM and HEV IgG were assessed with commercially available kits: the EIAgen HEV IgG and IgM kits (Adaltis, Casalecchio Di Reno, Italy) before 2012, and the Wantai HEV IgG and IgM enzyme-linked immunosorbent assay (Beijing Wantai Biologic Pharmacy Enterprise Co., Ltd, Beijing, China) since 2012. Diagnosis of hepatitis E required liver enzyme abnormalities and detectable HEV RNA in serum or fecal samples. Chronic HEV infection was defined by HEV viremia lasting >3 months (1).
Ribavirin monotherapy was proposed for infected immunocompromised patients beginning in May 2010, after publication of preliminary data reporting its efficacy in such patients (12). Assessment for administering ribavirin treatment was made on a case-by-case basis according to clinical and therapeutic context. Although there was no formal protocol, the general consensus was to consider ribavirin with the intent to complete treatment for the underlying disease rather than biologic criteria such as alanine aminotransferase levels or HEV viral loads. This study was approved by our institutional review board.
During 2003–2014, we identified 26 patients whose laboratory test results showed HEV replication. Clinical characteristics and treatments of patients with hematologic malignancies before or within the 6 months after the diagnosis of hepatitis E are summarized in Table 1 and in the Technical Appendix Table.
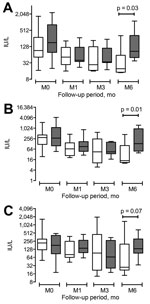
The diagnosis of HEV infection occurred a median of 10 (range 0–227) months after identification of the hematologic disorder. No persons in the study had traveled outside France during the year before hepatitis was diagnosed. Primary signs and symptoms were fever, diffuse or abdominal pain, vomiting, or asthenia; 16 patients were asymptomatic. Liver enzyme levels ranged from 1.5–100× the upper limit of normal (Figure). No patient had fulminant hepatitis. HEV RNA was detectable in the serum of all patients and in the fecal samples of 88% of patients (HEV strain genotype 3f in 73%, 3c in 23%, and 4b in 4%). Of 23 tested patients, 9 (36%) had HEV IgG and 13 (61%) had HEV IgM. This finding emphasizes the need to rely on HEV RNA detection rather than serologic results for HEV diagnosis in immunocompromised patients. HEV was diagnosed concomitantly to the onset (n = 2) or at relapse (n = 2) of the hematologic malignancy, which raises the hypothesis of an association between HEV infection and carcinogenesis (13).
The outcomes of both HEV infection and hematologic malignancies are summarized in Table 2. Twelve patients were treated with ribavirin after a median delay of 1 month (range 0.5–12 months) after the onset of hepatitis. Among the 14 patients with acute hepatitis E who did not receive ribavirin, 9 showed spontaneous clearance of HEV within the 2 months after diagnosis. The remaining 5 patients had prolonged HEV replication lasting >3 months, including 1 patient who had superimposed nonalcoholic steatohepatitis and had developed overt cirrhosis and refractory ascites. Immunosuppression withdrawal in 1 patient, who received allogeneic-SCT and in whom chronic hepatitis E developed, resulted in a severe transient incident of elevated liver enzymes, followed by spontaneous clearance of the virus.
Oral ribavirin was administered to 12 patients at a median dose of 800 mg/d (range 600–1,000 mg/d); 1 patient had chronic hepatitis. In all patients, HEV RNA became undetectable after 30 (range 10–60) days, and liver blood tests were normalized after 2.5 (range 1–12) months. Ribavirin was given for a median time of 3 (range 0.5–10) months. The heterogeneity of timing and duration of treatment likely reflected the progressive introduction of ribavirin into current practice and the specific context of several patients. In general, 2 consecutive negative PCRs during 1 month were required to discontinue ribavirin. Three patients had abnormal liver test results and HEV recurred within the month after ribavirin treatment was withdrawn, including 2 who received ribavirin for 1 month only. Definitive virologic response was obtained after ribavirin retreatment. Thus, the combination of a 3-month intake of ribavirin, 2 negative HEV blood RNAs at month 2 and 3, and negative RNA levels in stools at month 3 seems to be an appropriate approach.
Overall, aspartate aminotransferase and alanine aminotransferase serum levels were significantly higher at 6 months in patients who did not receive ribavirin compared with patients who did (p = 0.03 and p = 0.01, respectively; Technical Appendix Table). Ribavirin was well tolerated among 10 of the 12 recipients and did not require specific red blood cells or erythropoietin support. One patient, 84 years of age, became anemic while receiving 1,000 mg of ribavarin per day, but treatment was continued. Treatment was discontinued for another patient, 41 years of age, when pruritus developed after 2 weeks of therapy.
After SOT, chronic hepatitis develops in 60% of untreated HEV-infected patients (3). Here, we show that patients with hematologic malignancies are also at risk for chronic HEV infection. SOT patients showed a high response rate to ribavirin treatment (11). Because most hematologic malignancies required intensive immune treatments that should not be postponed, HEV infection could notably alter the therapeutic schedule of some patients (5,6). Cytotoxic treatments or SCT were postponed while awaiting HEV clearance for 5 patients, of whom 4 reached complete hematologic response. Among the 12 ribavirin-treated patients, 9 were able to complete the planned treatment that included SCT. Thus, although spontaneous but unpredictable HEV clearance could occur in some patients, therapeutic intervention is often mandatory to fully complete the therapeutic schedule of the underlying disease and to prevent chronic hepatitis. Confirming previous reports (11,12), a 3-month course of ribavirin induced complete and sustained HEV eradication in most if not all patients. Moreover, ribavirin may also decrease the risk for nosocomial HEV transmission among patients (14).
Our study has limitations. The retrospective nature prevented accurate assessment of the incidence of HEV infection among patients who have hematologic malignancies. Some obvious bias, including occurrence of HEV viremia without concomitant liver tests abnormalities or inability to ask patients about their exposure risk to HEV, also need further considerations.
By inducing a rapid clearance of HEV, ribavirin can prevent chronic hepatitis in patients with hematologic malignancies. Hepatitis E can induce chronic hepatitis and cirrhosis in patients with hematologic cancers; patients with liver enzyme abnormalities should be screened for HEV infection and a 3-month course of ribavirin proposed to avoid postponement in the treatment of patients who have the underlying disease as well as chronic hepatitis or cirrhosis.
Dr. Tavitian is a hematologist at the Institut Universitaire du Cancer de Toulouse. Her clinical research interests include infectious complications related to hematological malignancies, myeloproliferative neoplasia and acute lymphoblastic leukemia.
Acknowledgment
We thank the members of the Gaël Adolescent Espoir Leucémie and Association des Greffés de Moelle Osseuse de Midi-Pyrénées for their kind support to patients.
References
- Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin Microbiol Rev. 2014;27:116–38. DOIPubMedGoogle Scholar
- Hewitt PE, Ijaz S, Brailsford SR, Brett R, Dicks S, Haywood B, Hepatitis E virus in blood components: a prevalence and transmission study in southeast England. Lancet. 2014;384:1766–73.PubMedGoogle Scholar
- Kamar N, Selves J, Mansuy J-M, Ouezzani L, Péron J-M, Guitard J, Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358:811–7. DOIPubMedGoogle Scholar
- Dalton HR, Keane FE, Bendall R, Mathew J, Ijaz S. Treatment of chronic hepatitis E in a patient with HIV infection. Ann Intern Med. 2011;155:479–80. DOIPubMedGoogle Scholar
- Versluis J, Pas SD, Agteresch HJ, de Man RA, Maaskant J, Schipper MEI, Hepatitis E virus: an underestimated opportunistic pathogen in recipients of allogeneic hematopoietic stem cell transplantation. Blood. 2013;122:1079–86. DOIPubMedGoogle Scholar
- Tavitian S, Péron J-M, Huynh A, Mansuy J-M, Ysebaert L, Huguet F, Hepatitis E virus excretion can be prolonged in patients with hematological malignancies. J Clin Virol. 2010;49:141–4. DOIPubMedGoogle Scholar
- Halac U, Béland K, Lapierre P, Patey N, Ward P, Brassard J, Cirrhosis due to chronic hepatitis E infection in a child post-bone marrow transplant. J Pediatr. 2012 May;160(5):871–4.e1.
- Pfefferle S, Frickmann H, Gabriel M, Schmitz N, Günther S, Schmidt-Chanasit J. Fatal course of an autochthonous hepatitis E virus infection in a patient with leukemia in Germany. Infection. 2012;40:451–4. DOIPubMedGoogle Scholar
- Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269–79. DOIPubMedGoogle Scholar
- Geng Y, Zhang H, Huang W. J Harrison T, Geng K, Li Z, et al. Persistent hepatitis E virus genotype 4 infection in a child with acute lymphoblastic leukemia. Hepat Mon. 2014;14:e15618. DOIGoogle Scholar
- Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med. 2014;370:1111–20. DOIPubMedGoogle Scholar
- Kamar N, Rostaing L, Abravanel F, Garrouste C, Lhomme S, Esposito L, Ribavirin therapy inhibits viral replication on patients with chronic hepatitis E virus infection. Gastroenterology. 2010;139:1612–8. DOIPubMedGoogle Scholar
- Woolson KL, Forbes A, Vine L, Beynon L, McElhinney L, Panayi V, Extra-hepatic manifestations of autochthonous hepatitis E infection. Aliment Pharmacol Ther. 2014;40:1282–91.PubMedGoogle Scholar
- Mansuy J-M, Huynh A, Abravanel F, Recher C, Peron JM, Izopet J. Molecular evidence of patient-to-patient transmission of hepatitis E virus in a hematology ward. Clin Infect Dis. 2009;48:373–4. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleTable of Contents – Volume 21, Number 8—August 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christian Recher, Service d’Hématologie, Institut Universitaire du Cancer de Toulouse Oncopole, 1 avenue Irène Joliot-Curie, 31059 Toulouse CEDEX 9, France
Top