Volume 22, Number 10—October 2016
Synopsis
Outbreaks of Human Salmonella Infections Associated with Live Poultry, United States, 1990–2014
Abstract
Backyard poultry flocks have increased in popularity concurrent with an increase in live poultry–associated salmonellosis (LPAS) outbreaks. Better understanding of practices that contribute to this emerging public health issue is needed. We reviewed outbreak reports to describe the epidemiology of LPAS outbreaks in the United States, examine changes in trends, and inform prevention campaigns. LPAS outbreaks were defined as ≥2 culture-confirmed human Salmonella infections linked to live poultry contact. Outbreak data were obtained through multiple databases and a literature review. During 1990–2014, a total of 53 LPAS outbreaks were documented, involving 2,630 illnesses, 387 hospitalizations, and 5 deaths. Median patient age was 9 years (range <1 to 92 years). Chick and duckling exposure were reported by 85% and 38% of case-patients, respectively. High-risk practices included keeping poultry inside households (46% of case-patients) and kissing birds (13%). Comprehensive One Health strategies are needed to prevent illnesses associated with live poultry.
Salmonella species are zoonotic bacteria found in the intestinal tract of many animals, including cattle, pigs, horses, other mammals, reptiles, amphibians, and poultry (e.g., chickens, ducks, geese, and turkeys) (1). Nontyphoidal salmonellosis causes an estimated 1.2 million illnesses, 23,000 hospitalizations, and 450 deaths annually in the United States (2). Salmonella infection typically manifests as acute gastroenteritis that develops 12–72 hours after exposure. Young children, persons >65 years of age, and immunocompromised persons are at greater risk for serious complications, including septicemia, joint or brain infections, and death (3).
Although Salmonella is commonly transmitted through food, recent outbreaks have highlighted direct or indirect contact with animals as a frequent route of transmission (4). An estimated 11% of all Salmonella infections are attributed to animal exposure annually, with the highest rates of illness and death occurring among children (1). Since 2007, numerous outbreaks of human Salmonella infections linked to contact with animals and their environments have been investigated, including those involving contact with turtles, bearded dragons, African dwarf frogs, hedgehogs, and backyard poultry (5). Poultry can be persistent subclinical shedders and can appear healthy while shedding Salmonella bacteria (6). Zoonotic salmonellosis outbreak investigations require a One Health approach because they occur at the intersection of human and animal health (7).
In the United States, live poultry–associated salmonellosis (LPAS) outbreaks have been documented since 1955 (8). Historically, these outbreaks involved young children, occurred in the spring months around Easter, and were associated with birds obtained as pets (9). Baby poultry were often dyed bright colors, making them more attractive to young children. Currently, public health officials are identifying LPAS outbreaks linked to backyard poultry flocks that are affecting adults and children. Most of these outbreaks begin in the spring but continue over many months. The first multistate outbreak where the association with backyard flocks was recognized occurred in 2007 (10). Since that time, the popularity of backyard flocks has increased substantially (11). Most chicks sold for backyard flocks are produced by a network of mail-order hatcheries (9). Disease control guidance for hatcheries is provided by the US Department of Agriculture National Poultry Improvement Plan, which is a voluntary state, federal, and industry cooperative program aimed at eliminating certain diseases from poultry breeding flocks and hatcheries (12). We reviewed outbreak reports from 1990–2014 to describe the epidemiology of LPAS outbreaks in the United States, to identify changes in trends, and to identify practices of concern among case-patients to better inform future prevention campaigns.
We defined LPAS outbreaks as >2 culture-confirmed human Salmonella infections in the United States with a combination of epidemiologic, laboratory, or traceback evidence linking illnesses to live poultry contact. Data sources included PulseNet, the national molecular subtyping network for foodborne disease surveillance in the United States; the Centers for Disease Control and Prevention (CDC) Outbreak Response and Prevention Branch’s outbreak management database; and CDC’s National Outbreak Reporting System (13–15). Through these data sources, we collected outbreak summaries from 50 states and 4 US territories. Additionally, we conducted a literature review to identify any additional LPAS outbreaks that had not been reported to CDC. In January 2015, we searched PubMed without date or language restrictions and used combinations of the terms “salmonella,” “salmonellosis,” “outbreak,” “poultry,” and “United States.” To avoid including duplicate reports, we further reviewed outbreaks that occurred in the same year and for which identical Salmonella serotypes were reported.
A standardized live poultry exposure questionnaire was developed by officials at CDC, state and local health departments, and the National Poultry Improvement Plan. The questionnaire focused on patient demographics, baby and adult poultry contact, poultry purchases, flock management, and Salmonella awareness. The questionnaire was created in 2008, and since its creation, it has been administered to case-patients (or their parents/guardians) who were part of 21 multistate outbreak investigations during 2008–2013. To identify common patient characteristics and practices that might have increased the risk for Salmonella transmission from poultry to humans, we analyzed the results of these questionnaires by using SAS version 9.2 (SAS Institute, Cary, NC, USA).
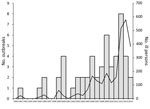
A total of 53 LPAS outbreaks were documented in the United States during 1990–2014 (Table 1); these 53 outbreaks were associated with 2,630 illnesses, 387 hospitalizations, and 5 deaths (Figure 1). Median outbreak size was 26 case-patients (range 4–363 case-patients). Approximately 77% (41/53) of outbreaks were multistate outbreaks.
The number of LPAS outbreaks reported annually has increased substantially in recent years (Figure 1). During 1990–2005, a total of 17 outbreaks were documented (1.06/year), with a median size of 12 case-patients per outbreak (range 4–53 case-patients). In comparison, during 2006–2014, a total of 36 outbreaks were documented (4/year), with a median size of 41 case-patients per outbreak (range 4–363 case-patients). The number of LPAS outbreaks peaked in 2012, with a total of 8 individual outbreaks. The 4 largest outbreaks occurred during 2012–2014.
Reported outbreak onset dates ranged from January to July. Most (80%) outbreaks began in the months of February, March, and April. Outbreak duration ranged from 1 to 12 months, with an average duration of 4.9 months.
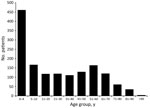
Montevideo was the serotype identified in 36% (19/53) of LPAS outbreaks, making it the most common Salmonella serotype reported. Among the Salmonella strains associated with LPAS outbreaks, 62% (38/61) were serogroup C1; serogroup B accounted for 16% (10/61), and serogroup C2 accounted for 13% (8/61). Serogroups C3, D1, and R were also reported. Additionally, 4 outbreaks consisted of multiple Salmonella serotypes. In the outbreaks with available information, 54% (1,026/1,898) of case-patients were male, and 46% (872/1,898) were female. Median case-patient age was 9 years (range <1 to 92 years); 31% (467/1,488) of case-patients were <5 years of age, and 42% (628/1488) were <10 years of age (Figure 2).
A total of 62% (511/822) of case-patients reported exposure to baby poultry (Table 2). Chick exposure was reported by 85% (434/511) of case-patients and duckling exposure by 38% (195/511). Among case-patients exposed to baby poultry, 62% reported exposure to only chicks (316/511), 15% (77/511) exposure to only ducklings, and 22% (118/511) exposure to both chicks and ducklings. Approximately 23% (117/582) of respondents reported contact with adult poultry. Among all outbreaks, the median time between purchase of poultry and illness onset was 17 days (range 1–672 days). Approximately 66% of case-patients reported <30 days between obtaining poultry and illness onset. However, 7% of case-patients reported >60 days between obtaining poultry and illness onset.
Among respondents with baby poultry exposure, 74% (276/373) reported that exposure occurred at the home. Approximately 76% (303/400) of respondents reported touching baby birds, 61% (227/373) reported touching the cage/coop of the baby birds, 49% (196/400) reported snuggling baby birds, and 13% (53/400) reported kissing baby birds.
Nearly 46% (188/413) of respondents reported keeping poultry inside the house. Of these, 22% (41/188) reported keeping live poultry in the living room, 12% (22/188) in the kitchen, 10% (18/188) in a bedroom, and 10% (18/188) in a bathroom. Approximately 52% of respondents reported owning poultry for <1 year. When asked if they were aware of a connection between poultry contact and Salmonella, 58% (167/290) of respondents reported that they were aware of the risk.
The number of LPAS outbreaks reported annually has increased substantially in recent years. Because only a small proportion of Salmonella infections are diagnosed and reported to public health departments, the actual number of illnesses in these outbreaks might be much larger with an estimated 29 additional infections going unreported for every reported case (2). These outbreaks are not only happening with increased frequency but are also generally affecting more persons. In addition, 62% of case-patients reported contact with baby chicks or ducklings, and 45% were <10 years of age. This finding is possibly attributable to the fact that children’s immune systems are not fully developed and that young children typically have poor hand hygiene practices. Most contact occurred at the patients’ home, and high-risk behaviors included keeping poultry inside the house and having close contact, such as holding, snuggling, or kissing poultry. These findings highlight the need for additional consumer education, especially on the risk for illness in children, the necessity for keeping live poultry outside of the home, and the recommendation to wash hands after coming in contact with live poultry.
Instead of being sold as novelty pets around the Easter holiday, chicks, ducklings, goslings, and turkey poults are now additionally being sold for backyard flocks; these birds can be purchased at agricultural feed stores across the United States. The practice of keeping backyard flocks of live poultry has gained popularity during the past decade (11). This increase is attributable to various reasons, including growing interest in local and organic food production, animal welfare concerns, environmental concerns, the desire to provide a learning experience for children, and the perception that local eggs are healthier and of better quality than store-bought eggs (34). In addition, backyard flocks are becoming increasingly common in urban and suburban areas (11). The fact that half of respondents to the live poultry questionnaire owned poultry for <1 year could signify that new owners might be unfamiliar with appropriate husbandry practices.
Most poultry sold for backyard flocks are produced by a core group of ≈20 mail-order hatcheries. These hatcheries sell more than 50 million chicks annually, and most distribute chicks nationwide (20). Distribution of baby poultry occurs through the US Postal Service, by which a small proportion are mailed directly to owners, whereas most baby poultry are sold to agricultural feed stores. Baby poultry are shipped in cardboard boxes that can contain 120 chicks, 60 ducklings, 32 goslings, or 80 turkey poults. One box may contain multiple species, and shipment can provide ample opportunity for cross-contamination. Increased shedding of Salmonella can occur when poultry are subjected to stressful conditions, such as transportation through the mail (6). The nationwide distribution as well as the opportunity for cross-contamination might help to explain the multistate distribution of outbreaks.
The serotypes identified in LPAS outbreaks are different from Salmonella serotypes, such as Enteritidis and Heidelberg, that are traditionally associated with foodborne poultry outbreaks (35,36). This finding might be attributable in part to differences in poultry that originate from mail-order hatcheries and commercial poultry hatcheries. Mail-order hatcheries typically operate on a much smaller scale, with more species and breeds of poultry sourced from breeding stock within their own farm or eggs from other mail-order hatcheries, which could explain the diversity of Salmonella serotypes identified in these outbreaks. In comparison, commercial poultry operations are typically larger scale, closed operations with 1 species and fewer breeds of bird on site (37).
The seasonality of these outbreaks might be attributable to the fact that most agricultural feed stores sell large numbers of chicks during spring or fall promotional events or “chick days.” These events provide additional opportunity for cross-contamination in the stores because of the increase in volume of chicks during these events. In addition to the increase in volume of chicks being sold in the spring, some households might keep chicks inside the home because of concerns that the chicks will not do well in cold weather.
Poultry can appear healthy and still shed Salmonella bacteria intermittently for extended periods of time (38). This intermittent shedding could contribute to the fact that some case-patients reported illness onset >1 year after poultry purchase. In addition, intermittent shedding could partially explain why recent outbreaks have been of a longer duration, some lasting up to 12 months.
The findings of this investigation are subject to several limitations. Smaller, single-state outbreaks might have been missed if they were not reported to National Outbreak Reporting System or not documented elsewhere. Additional outbreaks might have been missed if they were not detected by PulseNet or if, during the course of the investigation, public health practitioners did not ask case-patients about exposure to live poultry. Finally, results of the supplemental poultry questionnaires were only available for multistate outbreaks that occurred in 2008 or later. Case-patients from earlier outbreaks might have had different characteristics and had different types of poultry exposure in comparison to case-patients from the more recent outbreaks. Because our study relied on aggregated outbreak data, we could not calculate the relative magnitude of risk for different handling practices; therefore, we are unable to state which practices contribute the most to Salmonella transmission from live poultry to humans.
This review highlights the need for an integrated One Health response to LPAS outbreaks. One Health is defined as the collaborative effort of experts in multiple disciplines, including healthcare professionals, veterinarians, epidemiologists, and environmental scientists, working to attain optimal health for humans, animals, and the environment (7). Prevention and control efforts for LPAS outbreaks include interventions that target hatcheries, agricultural feed stores, health professionals, and consumers. Detailed recommendations for a comprehensive One Health prevention approach are available (9).
To prevent future outbreaks, the general public needs to be educated about the risk for LPAS. Persons need to be aware that healthy poultry can shed Salmonella intermittently, that persons need to wash their hands after contact with live poultry, that young children are at an increased risk for salmonellosis, and that poultry should never be allowed inside the house. Mail-order hatcheries, agricultural feed stores, public health officials, local and federal departments of agriculture, pediatricians, and veterinarians can all help to spread awareness about the association between live poultry and Salmonella infections. CDC has developed various educational resources that mail-order hatchery Web sites can link to (Figure 3). Posters and additional educational material can be displayed at points of sale (39). CDC has participated in a series of online consumer educational webinars with the US Department of Agriculture and other poultry interest groups (40). Healthcare providers can talk to parents about the risk for zoonotic Salmonella in children, especially if high-risk pets are in the home (41,42). The Journal of the American Veterinary Medical Association recently reported on the increased need for veterinarians who are willing to treat backyard poultry (43). Veterinarians have a unique opportunity to educate poultry owners about Salmonella prevention and control strategies (9).
Poultry are acquiring a new position in many households. Instead of being treated as production animals, they are increasingly being considered household pets. However, recurring LPAS outbreaks highlight the need for strategies to prevent human illnesses associated with live poultry contact through a comprehensive One Health approach involving human, animal, and environmental health.
Dr. Basler completed this analysis while he was an Epidemic Intelligence Service officer through the Centers for Disease Control and Prevention. Currently, he is working as a veterinary epidemiologist with the Outbreak Response and Prevention Branch of the Division of Foodborne, Waterborne, and Environmental Diseases, National Center for Emerging and Zoonotic Infectious Diseases, CDC. His primary research interests include foodborne and enteric zoonotic outbreak investigations.
Acknowledgment
We would like to thank the mail-order hatchery and feed story industries, many state and local health departments, departments of agriculture, the US Department of Agriculture, and the US Centers for Disease Control and Prevention staff for their contributions to this public health issue.
References
- Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis. 2012;54(Suppl 5):S472–9.DOIPubMedGoogle Scholar
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15.DOIPubMedGoogle Scholar
- Giannella RA. Salmonella. In: Baron S, ed. Medical microbiology. 4th ed. Galveston (TX): University of Texas Medical Branch at Galveston; 1996.
- Steinmuller N, Demma L, Bender JB, Eidson M, Angulo FJ. Outbreaks of enteric disease associated with animal contact: not just a foodborne problem anymore. Clin Infect Dis. 2006;43:1596–602.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Gastrointestinal (enteric) diseases from animals [cited 2014 Sep 16]. http://www.cdc.gov/zoonotic/gi
- Gast RK, Holt PS. Persistence of Salmonella enteritidis from one day of age until maturity in experimentally infected layer chickens. Poult Sci. 1998;77:1759–62.DOIPubMedGoogle Scholar
- American Veterinary Medical Association. One Health—it’s all connected [cited 2014 Sep 16]. https://www.avma.org/KB/Resources/Reference/Pages/One-Health.aspx
- Anderson AS, Bauer H, Nelson CB. Salmonellosis due to Salmonella typhimurium with Easter chicks as likely source. J Am Med Assoc. 1955;158:1153–5.DOIPubMedGoogle Scholar
- Behravesh CB, Brinson D, Hopkins BA, Gomez TM. Backyard poultry flocks and salmonellosis: a recurring, yet preventable public health challenge. Clin Infect Dis. 2014;58:1432–8.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Multistate outbreaks of Salmonella infections associated with live poultry—United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:25–9.PubMedGoogle Scholar
- Beam A, Garber L, Sakugawa J, Kopral C. Salmonella awareness and related management practices in U.S. urban backyard chicken flocks. Prev Vet Med. 2013;110:481–8.DOIPubMedGoogle Scholar
- US Department of Agriculture. National Poultry Improvement Plan: helping you, the poultry breeder, prevent disease [cited 2014 Sep 16]. http://www.aphis.usda.gov/publications/animal_health/content/printable_version/HelpingYouPoultryBreeder-PA1708-FinalJuly09.pdf
- Centers for Disease Control and Prevention. PulseNet [cited 2014 Sep 16]. http://www.cdc.gov/pulsenet
- Centers for Disease Control and Prevention. Outbreak Response and Prevention Branch [cited 2014 Sep 16]. http://www.cdc.gov/ncezid/dfwed/orpb
- Centers for Disease Control and Prevention. The National Outbreak Reporting System (NORS) [cited 2015 Apr 14]. http://www.cdc.gov/nors
- Centers for Disease Control (CDC). Salmonella hadar associated with pet ducklings—Connecticut, Maryland, and Pennsylvania, 1991. MMWR Morb Mortal Wkly Rep. 1992;41:185–7.PubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Salmonella serotype Montevideo infections associated with chicks—Idaho, Washington, and Oregon, spring 1995 and 1996. MMWR Morb Mortal Wkly Rep. 1997;46:237–9.PubMedGoogle Scholar
- Bidol S, Stobierski M, Robinson-Dunn B, Massey J, Hall W, Boulton M, ; Centers for Disease Control and Prevention (CDC). Salmonellosis associated with chicks and ducklings—Michigan and Missouri, Spring 1999. MMWR Morb Mortal Wkly Rep. 2000;49:297–9.PubMedGoogle Scholar
- Wilkins MJ, Bidol SA, Boulton ML, Stobierski MG, Massey JP, Robinson-Dunn B. Human salmonellosis associated with young poultry from a contaminated hatchery in Michigan and the resulting public health interventions, 1999 and 2000. Epidemiol Infect. 2002;129:19–27.DOIPubMedGoogle Scholar
- Gaffga NH, Barton Behravesh C, Ettestad PJ, Smelser CB, Rhorer AR, Cronquist AB, Outbreak of salmonellosis linked to live poultry from a mail-order hatchery. N Engl J Med. 2012;366:2065–73.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Three outbreaks of salmonellosis associated with baby poultry from three hatcheries—United States, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:273–6.PubMedGoogle Scholar
- Hedican E, Smith K, Jawahir S, Scheftel J, Kruger K, Birk R, ; Centers for Disease Control and Prevention (CDC). Multistate outbreaks of Salmonella infections associated with live poultry—United States, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:25–9.PubMedGoogle Scholar
- Loharikar A, Vawter S, Warren K, Deasy M III, Moll M, Sandt C, Outbreak of human Salmonella Typhimurium infections linked to contact with baby poultry from a single agricultural feed store chain and mail-order hatchery, 2009. Pediatr Infect Dis J. 2013;32:8–12.DOIPubMedGoogle Scholar
- Loharikar A, Briere E, Schwensohn C, Weninger S, Wagendorf J, Scheftel J, Four multistate outbreaks of human Salmonella infections associated with live poultry contact, United States, 2009. Zoonoses Public Health. 2012;59:347–54.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention (CDC). Notes from the field: multistate outbreak of Salmonella Altona and Johannesburg infections linked to chicks and ducklings from a mail-order hatchery - United States, February-October 2011. MMWR Morb Mortal Wkly Rep. 2012;61:195.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Altona and Salmonella Johannesburg infections linked to chicks and ducklings [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/altona-baby-chicks/index.html
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Hadar infections linked to live poultry in backyard flocks (final update) [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/hadar-live-poultry-07-12/index.html
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Montivedeo infections linked to live poultry in backyard flocks (final update) [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/montevideo-06-12/index.html
- Centers for Disease Control and Prevention (CDC). Notes from the field: Multistate outbreak of Salmonella infantis, newport, and lille infections linked to live poultry from a single mail-order hatchery in Ohio—March-September, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:213.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella infections linked to live poultry in backyard flocks (final update) [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/live-poultry-05-12/index.html
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella infections linked to live poultry (final update) [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/live-poultry-04-13/index.html
- Basler C, Forshey TM, Machesky K, Erdman MC, Gomez TM, Nguyen TA, ; Centers for Disease Control and Prevention (CDC). Multistate outbreak of human Salmonella infections linked to live poultry from a mail-order hatchery in Ohio—March-September 2013. MMWR Morb Mortal Wkly Rep. 2014;63:222.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multistate outbreak of human Salmonella Typhimurium infections linked to live poultry in backyard flocks (final update) [cited 2014 Sep 18]. http://www.cdc.gov/salmonella/typhimurium-live-poultry-04-13/index.html
- US Department of Agriculture. Urban chicken ownership in four U.S. cities [cited 2014 Sep 16]. http://www.aphis.usda.gov/animal_health/nahms/poultry/downloads/poultry10/Poultry10_dr_Urban_Chicken_Four.pdf
- Braden CR. Salmonella enterica serotype Enteritidis and eggs: a national epidemic in the United States. Clin Infect Dis. 2006;43:512–7.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Multistate outbreak of multi-drug-resistant Salmonella Heidelberg infections linked to Foster Farms brand chicken (final update) [cited 2016 Feb 25]. http://www.cdc.gov/salmonella/heidelberg-10-13/index.html
- MacDonald J. The economic organization of U.S. broiler production. Economic Information Bulletin No. (EIB-38).Washington: US Department of Agriculture; 2008. http://www.ers.usda.gov/publications/eib-economic-information-bulletin/eib38.aspx
- Van Immerseel F, De Buck J, Pasmans F, Bohez L, Boyen F, Haesebrouck F, Intermittent long-term shedding and induction of carrier birds after infection of chickens early posthatch with a low or high dose of Salmonella enteritidis. Poult Sci. 2004;83:1911–6.DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Gastrointestinal (enteric) diseases educational materials and other resources [cited 2014 Sep 16]. http://www.cdc.gov/zoonotic/gi/education.html
- US Department of Agriculture. Biosecurity for the birds [cited 2014 Sep 16]. http://www.aphis.usda.gov/animal_health/birdbiosecurity
- Centers for Disease Control and Prevention. Love the pets, not the germs: CDC update on enteric zoonoses [cited 2014 Sept 16]. http://emergency.cdc.gov/coca/calls/2014/callinfo_071714.asp
- Pickering LK, Marano N, Bocchini JA, Angulo FJ; Committee on Infectious Diseases. Exposure to nontraditional pets at home and to animals in public settings: risks to children. Pediatrics. 2008;122:876–86.DOIPubMedGoogle Scholar
- Kaiser J. Meet your new neighbors: chickens are moving from the henhouse to the backyard and looking for veterinary care. J Am Vet Med Assoc. 2013;243:458–63.DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 22, Number 10—October 2016
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Colin Basler, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop A38, Atlanta, GA 30329-4027, USA
Top