Volume 23, Number 4—April 2017
Dispatch
Zika Virus Seroprevalence, French Polynesia, 2014–2015
Abstract
During 2013–2014, French Polynesia experienced an outbreak of Zika virus infection. Serosurveys conducted at the end of the outbreak and 18 months later showed lower than expected disease prevalence rates (49%) and asymptomatic:symptomatic case ratios (1:1) in the general population but significantly different prevalence rates (66%) and asymptomatic:symptomatic ratios (1:2) in schoolchildren.
Zika virus (family Flaviviridae, genus Flavivirus), an arthropodborne pathogen, is transmitted to humans by Aedes spp. mosquitoes, but nonvectorborne transmission (i.e., maternofetal and sexual transmission and transmission via blood transfusion) has also been reported (1). Infection by Zika virus most commonly causes mild disease consisting of fever, rash, arthralgia, headache, and conjunctivitis (1), but severe neurologic complications, such as Guillain-Barré syndrome in adults (2) and microcephaly in fetuses and newborns (3), have been described.
Zika virus emerged for the first time in 2007 on Yap Island, Federated States of Micronesia, in the Pacific region (4). Six years later, Zika virus caused an explosive outbreak in French Polynesia (5), and the virus then spread across the Pacific region (6). During October 2013–April 2014 in French Polynesia, an estimated 32,000 persons (11.5% of the population) visited healthcare facilities because of clinical symptoms suggestive of Zika virus infection (1,7). A retrospective serosurvey conducted on blood collected from donors before the outbreak confirmed that Zika virus had not previously circulated in French Polynesia (8). We conducted a study to assess Zika virus seroprevalence among the French Polynesia population after the virus emerged in the country.
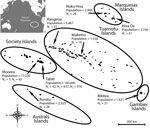
French Polynesia comprises 119 islands distributed among 5 archipelagos (Society, Tuamotu, Marquesas, Australs, and Gambier). The population of ≈270,000 inhabitants lives on 74 islands (2012 census; http://www.ispf.pf/docs/default-source/publi-pr/POP_LEGALE_2012_PF.pdf?sfvrsn=2). During February and March 2014, we conducted a cluster sampling among the general population living in the 5 archipelagos. We randomly recruited a total of 196 participants on the most inhabited islands of each archipelago: Tahiti and Moorea (Society), Rangiroa and Makemo (Tuamotu), Nuku Hiva and Hiva Oa (Marquesas), Rurutu (Australs), and Rikitea (Gambier) (Figure). Because >85% of the inhabitants of French Polynesia live on the Society Islands, we conducted a second cluster sampling among 700 participants recruited on Tahiti and Moorea during September–November 2015. In addition, 476 schoolchildren initially recruited for a dengue serosurvey on Tahiti during May and June 2014 were included in the study.
All participants were asked to declare whether they had clinical manifestations suggestive of past Zika infection. Adults provided written informed consent before enrollment, and parents or guardians gave consent for their children. Participants’ blood samples and personal data were anonymized before processing, and the study was approved by the Ethics Committee of French Polynesia (no. 60/CEPF 06/27/2013).
We used a recombinant antigen–based indirect ELISA to detect Zika virus IgG in blood samples collected in 2014 from the general population and schoolchildren (8). We also tested serum samples from the general population by microsphere immunoassay (MIA), using the same recombinant antigens as for the ELISA (2,8). Among the 196 serum samples from the general population, 80% tested positive for Zika virus IgG by both assays (κ = 0.51, indicating good agreement between ELISA and MIA results). Blood samples collected in 2015 were tested by MIA only.
Zika virus seroprevalence rates and proportions of asymptomatic infections were 49% (95% CI 42%–57%) and 43% (95% CI 33%–53%), respectively, for participants from the general population sampled in 2014 and 66% (95% CI 60%–71%) and 29% (95% CI 24%–34%), respectively, for schoolchildren sampled in 2014 (Table). Seroprevalence rates and proportions of asymptomatic infections were 22% (95% CI 16%–28%) and 53% (95% CI 45%–61%), respectively, for participants recruited in 2015 (Table).
During the October 2013–April 2014 Zika infection outbreak in French Polynesia, ≈11.5% of the population sought medical care for symptoms suggestive of Zika infection (1,7); however, serosurveys at the end of the outbreak showed a Zika virus seroprevalence rate of 49% (95% CI 42%–57%), suggesting that most infected persons did not seek medical care. The finding that 43% (95% CI 33%–53%) of the participants had Zika virus IgG without self-reported symptoms reflects an estimated asymptomatic to symptomatic ratio of 1:1. These results suggest that infected persons did not consult medical care staff because the infection was mild or asymptomatic, as previously described (10). Of the 5 French Polynesia archipelagos, the Society Islands had the lowest seroprevalence rate (37% [95% CI 26%–47%]) and the Australs–Gambier Islands the highest (59% [95% CI 39%–80%]); however, seroprevalence among the archipelagos did not differ substantially, suggesting that no matter their location, study participants had similar Zika virus transmission exposure.
Eighteen months after the end of the outbreak, the Zika virus seroprevalence rate and proportion of asymptomatic infections among 700 persons on the Society Islands were 22% (95% CI 16%–28%) and 53% (95% CI 45%–61%), respectively, not substantially different from those during the first cluster sampling in the same islands (37% [95% CI 26%–47%] and 56% [95% CI 33%–79%], respectively). The finding that the Zika virus seroprevalence rate did not increase between the 2 sampling periods suggests that the virus did not actively circulate after the end of the outbreak. In contrast, the decrease in the Zika virus seroprevalence rate, even if not significant, suggests that Zika virus IgG titers may drop over time.
Within 2 months after the end of the outbreak, the Zika virus seroprevalence rate among schoolchildren (6–16 [median 11] years of age) on Tahiti was substantially higher than that among the general population (4–88 [median 43] years of age) (66% [95% CI 60%–71%] vs. 22% [95% CI 16%–28%], respectively). In contrast, the proportion of asymptomatic Zika virus infections was substantially lower among schoolchildren (29% [95% CI 24%–34%]) than among the general population (53% [95% CI 45%–61%]). Dengue virus (DENV) may provide cross-protection against Zika infection; thus, the higher DENV IgG seroprevalence among adults may explain why fewer adults than children were infected by Zika virus (8,11–13). The lower asymptomatic rate in children may have 2 additional explanations: the reporting of symptoms among children may have been compounded by the relatively higher frequency of febrile rash illness due to other viral infections, and sampling among children was conducted at the tail end of the outbreak, so they would likely remember symptoms more clearly than the population surveyed 18 months after the outbreak.
In the 3 groups tested, no difference was seen by sex in the seroprevalence rate or the proportion of asymptomatic infections. However, because the sampling scheme was not initially designed to compare data by sexes, data could not be extrapolated to the population level.
Our findings show that <50% of the population of French Polynesia had detectable Zika virus IgG. This seroprevalence rate is much lower than the 86% attack rate estimated by Kucharski et al. (14) using a model that assumed the French Polynesia population was 100% susceptible to Zika virus infection. However, in a setting where DENVs are highly prevalent (8), the possibility of cross-protecting immunity preventing infection from Zika virus (12,13) cannot be excluded. The attack rate and the asymptomatic:symptomatic ratio in French Polynesia were also lower than those described for the 2007 outbreak on Yap Island (73% and 4:1, respectively) (4); this finding supports the perception that the drivers of Zika virus transmission vary depending on geographic context. For other flaviviruses, such as DENV, previous model-based studies showed that the herd immunity threshold required to block viral transmission is ≈50%–85% (15). Thus, if Zika virus has the same epidemiologic characteristics as DENV, the seroprevalence rate of 49% would not be sufficient to prevent another outbreak.
Dr. Aubry is a research scientist at the Institut Louis Malardé, Tahiti, French Polynesia. Her research interests include arboviruses epidemiology and genetic evolution in the Pacific region.
Acknowledgments
We are grateful to Eliane Mama, Sylvia Fontanel, and Jean-Paul Pescheux for their help in collecting blood samples and to Teheipuaura Mariteragi-Helle for her technical support. We thank the administrators and health authorities of each French Polynesia archipelago and the mayors, municipal staff, practitioners, and nurses in the islands selected for the study for their support in recruiting participants and sending blood samples to the Institut Louis Malardé. We also thank the minister for education of French Polynesia; the Direction de l’enseignement primaire and the Direction de l’enseignement secondaire in French Polynesia; and the directors, teachers and nurses of the elementary and junior high schools selected for the study for their participation in recruiting schoolchildren and collecting blood samples. We especially thank all of the participants.
This study received funding from the Contrat de Projet Etat-Pays (convention no. 7331/MSS/DSP du 31/08/12 modifiée) and from the French Government’s Investissement d’Avenir Programme (Labex Integrative Biology of Emerging Infectious Diseases, IBEID, grant no. ANR-10-LABX-62-IBEID).
References
- Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9. DOIPubMedGoogle Scholar
- Cauchemez S, Besnard M, Bompard P, Dub T, Guillemette-Artur P, Eyrolle-Guignot D, et al. Association between Zika virus and microcephaly in French Polynesia, 2013-15: a retrospective study. Lancet. 2016;387:2125–32. DOIPubMedGoogle Scholar
- Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. DOIPubMedGoogle Scholar
- Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry A-L, Mallet H-P, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085–6. DOIPubMedGoogle Scholar
- Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595–6. DOIPubMedGoogle Scholar
- Mallet HP, Vial AL, Musso D. Bilan de l’épidémie à virus Zika survenue en Polynésie française entre octobre 2013 et mars 2014. De la description de l’épidémie aux connaissances acquises après l’évènement. Bulletin épidémiologique hebdomadaire. 2016;20–21:367–73.
- Aubry M, Finke J, Teissier A, Roche C, Broult J, Paulous S, et al. Seroprevalence of arboviruses among blood donors in French Polynesia, 2011-2013. Int J Infect Dis. 2015;41:11–2. DOIPubMedGoogle Scholar
- Spiegel A, Moulia-Pelat J, Daumerie D, Merlin M, Baudon D. Le sondage en grappe type OMS : méthodes pratiques en épidémiologie descriptive. Med Afr Noire. 1989;36:740–5.
- Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014;19:2pii=0761.
- Chungue E, Marché G, Plichart R, Boutin JP, Roux J. Comparison of immunoglobulin G enzyme-linked immunosorbent assay (IgG-ELISA) and haemagglutination inhibition (HI) test for the detection of dengue antibodies. Prevalence of dengue IgG-ELISA antibodies in Tahiti. Trans R Soc Trop Med Hyg. 1989;83:708–11. DOIPubMedGoogle Scholar
- Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016;17:1102–8. DOIPubMedGoogle Scholar
- Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113:7852–7. DOIPubMedGoogle Scholar
- Kucharski AJ, Funk S, Eggo RM, Mallet H-P, Edmunds WJ, Nilles EJ. Transmission dynamics of Zika virus in island populations: a modelling analysis of the 2013–14 French Polynesia outbreak. PLoS Negl Trop Dis. 2016;10:e0004726. DOIPubMedGoogle Scholar
- Johansson MA, Hombach J, Cummings DA. Models of the impact of dengue vaccines: a review of current research and potential approaches. Vaccine. 2011;29:5860–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleTable of Contents – Volume 23, Number 4—April 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Maite Aubry, Institut Louis Malardé, PO Box 30, 98713 Papeete, Tahiti, French Polynesia
Top