Volume 24, Number 3—March 2018
Research
Prospective Observational Study of Incidence and Preventable Burden of Childhood Tuberculosis, Kenya
Substantial progress has been made in the fight against tuberculosis (TB); however, new approaches are needed to achieve the current target set by the World Health Organization (WHO) to reduce TB incidence to 90% of 2016 levels by 2035 (1). A key element of WHO’s End TB Strategy is the prioritization of preventive treatment (2). However, the preventable burden of childhood TB has not been quantified in prospective epidemiologic studies, and globally, only an estimated 7% of eligible children received isoniazid chemoprophylaxis in 2015 (1).
Diagnosis of TB is more challenging in children than in adults (3). In low-resource settings, where TB burden is highest, diagnosis often relies on poorly validated clinical algorithms (4). As a result, adequate surveillance data are lacking, and published estimates of the global childhood TB burden vary widely (1,5–11). High-quality prospective data on the TB burden and case detection rate (CDR) in children are recognized priorities (8,11,12), and population-level data showing the preventable burden of childhood TB might reinforce the public health case for chemoprophylaxis in children. We designed the Kilifi Improving Diagnosis and Surveillance of Childhood TB (KIDS TB) Study to estimate the incidence, CDR, risk factors, and preventable burden of childhood TB in Kenya.
Study Sites
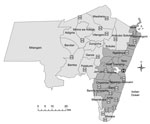
The study took place at Coast Provincial General Hospital (CPGH) and Kilifi County Hospital (KCH) in Coast Province, Kenya. CPGH provides primary and secondary care to the city of Mombasa and tertiary services for Coast Province. KCH is nested within the Kilifi Health and Demographic Surveillance System (KHDSS) (13), which covers a predominantly rural area of 891 km2 that in March 2011 was home to 261,919 residents in 29,970 households; two thirds of pediatric admissions to KCH during the study period were derived from this system. Three other health facilities in the KHDSS provide TB smear microscopy; 12 clinics are designated TB treatment centers (Figure 1). Because of resource constraints, contact tracing was not routine and isoniazid chemoprophylaxis not available at the time of the study, despite the inclusion of these steps in national TB guidelines.
Participants
We established a system of enhanced passive and active childhood TB surveillance. In the passive case–detection arm, we prospectively recruited all children <15 years of age who were seen at KCH or CPGH during August 2009–July 2011 for an unexplained persistent cough for >2 weeks, pneumonia not responding to antibiotics, unexplained fever for >2 weeks, unexplained progressive weight loss or failure to thrive for >4 weeks, close contact with a person with TB, or clinical suspicion of TB for any other reason. Study clinicians and clinicians from the hospital and surrounding clinics were trained in the symptoms and signs of a range of TB presentations (Technical Appendix Table 1). We excluded children with an established alternative diagnosis that explained all the clinical features as well as children already on TB treatment for >2 weeks at presentation. In the active case–detection arm, we recruited KHDSS-resident children <5 years of age sharing a household with persons with new cases of smear-positive pulmonary TB.
Clinical Procedures
All children underwent a similar structured history and examination, chest radiography, and tuberculin skin testing according to WHO guidelines (14) (online Technical Appendix). Children who were able to expectorate provided up to 3 spontaneous sputum samples. Sputum induction was performed on the remainder (14). Further investigations including extrapulmonary or repeat sputum sampling were performed at the discretion of the clinical team caring for the patient. Provider-initiated testing and counseling for HIV was performed according to national guidelines.
We classified children as having confirmed TB, highly probable TB, possible TB, or not TB (TB excluded) according to clinical, radiologic, and microbiological findings, based closely on stringent published definitions (Technical Appendix Table 2) (15,16). For comparison, we also applied other published clinical definitions to our dataset (Technical Appendix). Treatment protocols followed national guidelines. Children were followed up for 6 months or until a diagnosis of TB could be confidently excluded.
Laboratory Methods
Acid-fast bacilli microscopy and mycobacterial culture using the BACTEC MGIT system (BD Diagnostics, Sparks, MD, USA) were performed according to standard protocols (17). Positive cultures were further characterized using the BD MGIT TBc Identification Test (BD Diagnostics) and Hain Genotype line probe assays (Hain Lifescience GmbH, Nehren, Germany), including isoniazid and rifampin drug-susceptibility testing. We performed the Xpert MTB/RIF assay version G4 (Cepheid, Sunnyvale, CA, USA) at the end of the study on specimens from all children treated for confirmed, highly probable, or possible TB as well as from children for whom a TB diagnosis had been excluded. Laboratory procedures were externally monitored using the United Kingdom National External Quality Assessment Service’s quality-assurance scheme (http://ukneqas.org.uk).
Incidence Estimates
We used clinical data from KCH and event data from KHDSS to compile for every KHDSS-resident child a series of chronological time-span records representing the periods between consecutive birth, migration, enumeration, hospital presentation, or death events during the study period. We split these periods of observation by age category and estimated crude TB incidence rates as the total number of new TB cases identified (by both active and passive case detection) divided by the total person-years of observation in each age stratum. We compared estimates generated using the study case definitions with incidence estimates derived by applying other published clinical definitions of childhood TB to our dataset (Technical Appendix).
Estimating the CDR
Crude incidence estimates assume all incident cases among KHDSS residents are captured by the study; however, hospital-based surveillance of childhood illnesses is known to be insensitive in this setting (18–20). We defined the CDR as the proportion of KHDSS-resident TB cases captured by the study. Because the actual number of children with TB is unknown, we used 3 different methods to estimate the CDR independently (detailed description in Technical Appendix).
TB Notification Data
We linked clinical data with National Tuberculosis Programme notification data and KHDSS census data. We estimated the CDR as 1) the proportion of KHDSS-resident smear-positive childhood TB cases reported to the National Tuberculosis Programme that were captured by passive case detection at KCH, and 2) the proportion of children’s household contacts of new smear-positive pulmonary TB cases captured by active contact tracing.
Hospital-Based Mortality Surveillance
We linked KHDSS vital status data with KCH admission data. We then calculated the proportion of all childhood deaths in the KHDSS area captured at KCH during the study period.
Verbal Autopsy
By using disease-specific mortality data from a contemporaneous verbal autopsy study of all deaths within the KHDSS (21), we estimated the proportion of childhood TB deaths captured by our study. Because the number of child TB cases diagnosed by verbal autopsy is small and healthcare-seeking behavior is usually determined by clinical features rather than diagnosis per se (20,22), we also estimated the CDR as the proportion of children who died having clinical features of suspected TB that were captured by the study.
To derive the most conservative estimates of the actual annual incidence of childhood TB, we divided crude incidence rates by the highest CDR estimate. We modeled the likely number of incident confirmed or highly probable TB (CHPTB) cases among children nationally by multiplying the total number of adult cases reported in Kenya in 2010 (23) by the ratio of child-to-adult cases in the KHDSS, assuming a similar ratio and adult CDR nationally. We then used denominator population data from the national census (24) to estimate the national incidence of childhood TB.
Risk Factors for Childhood TB
We explored risk factors for childhood TB in a nested case–control analysis of children with CHPTB (cases) and children for whom TB was excluded (controls). To mitigate ascertainment bias in analysis of TB contact history, we excluded the small minority of children identified through active contact tracing. For each association, we derived crude odds ratios (ORs) and 95% CIs. We then included in a multivariable logistic regression model those variables with at least a weak association with TB in the univariable analysis (likelihood ratio test; p<0.1) and presented adjusted ORs and 95% CIs.
By using the number of KHDSS-resident adult cases reported to the National Tuberculosis Programme during the study period and the mean number of close contacts <5 years of age per case (25), we estimated the prevalence of household exposure to a person with confirmed TB among KHDSS-resident children <5 years of age. Using the contact status of CHPTB cases detected in the study, the child years at risk derived from the KHDSS census, and the exposure prevalence, we estimated the incidence of TB among contacts and noncontacts. The population attributable fraction for contact with a person with confirmed TB was calculated from the ensuing incidence rate ratio (IRR) and the exposure prevalence (p) by calculating p(IRR − 1)/1 + p(IRR − 1) (Technical Appendix).
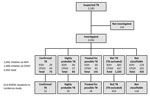
We identified 2,183 children with suspected TB during the study period and summarized patient enrollment and diagnostic assignments (Figure 2). We excluded 141 (6%) children who died, were discharged, or were lost to follow-up before their diagnostic workups, including specimen collection for mycobacterial culture, could be completed (Figure 2). We summarized baseline clinical characteristics of the remaining 2,042 children included in the analyses (Table 1).
Crude Incidence Estimates
We determined crude, hospital-based, age-specific incidence rates based on the study definitions (Table 2). The incidence of all childhood TB was 30.2 (95% CI 23.6–38.0) cases/100,000 children/year. The incidence of CHPTB was 18.4 (95% CI 13.4–24.7) cases/100,000 children/year; this estimate was very similar to that derived by retrospectively applying to our data consensus definitions of definite or probable TB that were published after completion of our study (26) (20.5 [95% CI 15.2–27.1]/100,000/year). Both figures are at the lower end of the range of estimates derived using published clinical definitions, which vary >30-fold (2.9–91.7/100,000/year) (Table 3).
CDR and Adjusted Incidence Estimates
CDR estimates derived using TB notifications, KHDSS census data, and verbal autopsy ranged from 0.2 to 0.35 (Table 4), substantially lower than the estimated CDR of 0.82 for adults in Kenya (41). Hospital-based mortality surveillance provided the largest and most precise estimate of the CDR (0.35 [95% CI 0.31–0.40]), so we used this to derive the most conservative estimates of the actual community incidence of childhood TB (Table 5). After adjustment for CDR, the incidence of CHPTB and all TB among children in the KHDSS was 53 (95% CI 38–71) and 86 (95% CI 67–109) cases/100,000/year, respectively.
Implications for the National Incidence of Childhood TB
During August 2009–July 2011, a total of 678 new cases of adult TB were reported to the National Tuberculosis Programme, and an estimated 126 new CHPTB cases were reported in children (Table 5) among KHDSS residents. Nationally 89,883 adult and 5,721 child TB cases were reported in 2010 (41) among a population that includes ≈17.6 million children <15 years of age (24). Applying the ratio of adult-to-child TB cases in the KHDSS to the national caseload yields an estimated 16,704 new CHPTB cases among children <15 years of age nationally in 2010, suggesting a national childhood TB CDR of 29% and incidence of 95 cases/100,000 children/year (Technical Appendix Table 3).
Risk Factors for Childhood TB
We summarized associations of CHPTB and important putative risk factors (Table 6). A history of known close TB contact at presentation was strongly associated with CHPTB, with an effect gradient according to the contacts’ smear status, proximity, relationship, and number (Technical Appendix Table 4). No child case-patients with a close TB contact had received isoniazid chemoprophylaxis. We observed a weaker association with HIV and in young children with severe malnutrition but no association between the presence of a bacillus Calmette-Guérin (BCG) vaccination scar and TB, although power to detect an effect was low because of the small proportion of children without a BCG vaccination scar.
Preventable TB Burden among Child Household TB Contacts
Among KHDSS-resident children <5 years of age, an estimated 1,259 were close contacts of adults with new TB cases reported during the study period. The incidence of CHPTB was 596 cases/100,000/year among children with a close TB contact and 17 cases/100,000/year among those without a close TB contact, yielding a 49% population attributable fraction for having a recent and known TB contact (Technical Appendix Table 5).
This study provides rare prospective empiric data on the TB incidence and CDR among children <15 years of age in Kenya, a country with a high TB burden, and is one of few prospective incidence studies globally (3). This community-based study was nested in a demographic surveillance survey, underpinned by enhanced active and passive surveillance, mycobacterial culture facilities, and linked hospital, demographic, notification, and verbal autopsy data. We used a hierarchical diagnostic classification in keeping with recommendations for childhood TB surveillance and research (26,35). A comprehensive algorithm of clinical, radiologic, and laboratory investigations combined with careful follow-up of children enrolled in the KIDS TB Study ensured diagnostic classifications were optimized within the limitations of currently available diagnostic tools.
Although the diagnosis of confirmed TB has the highest specificity, the poor sensitivity of mycobacterial culture for childhood TB diagnosis means that incidence estimates based only on confirmed cases will underestimate the actual disease burden. Conversely, including possible TB cases in the numerator might overestimate incidence. Most children in the highly probable TB group probably did have TB, given the stringent diagnostic criteria, and although the sensitivity of this classification is not perfect, it probably captured many of the actual cases of active TB for which culture confirmation was not obtained. We therefore used a combination of confirmed or highly probable TB (CHPTB) as the measure most likely to optimize sensitivity and specificity for estimation of childhood TB incidence.
Compared with estimates based on published clinical definitions, our measure of CHPTB incidence is among the most conservative, similar to the estimate obtained by retrospectively applying more recent consensus definitions for research (26). Even after inclusion of all TB cases, our measure remained among the lowest, suggesting that many published clinical definitions would overdiagnose TB in this and similar settings were they to be applied routinely in clinical practice. The huge range in incidence estimates derived using different case definitions emphasizes the difficulty in interpreting existing disease burden data and the need for high-quality prospective incidence studies to improve disease burden estimates.
Robust community incidence estimates depend on high-quality diagnosis to minimize misclassification as well as a high CDR. Broad screening criteria for all children admitted to hospital with any features of suspected TB, plus active case detection through contact tracing, ensured that case ascertainment at KCH was optimized. Nevertheless, the social, financial, and geographic barriers to obtaining hospital care in this setting mean that many ill KHDSS-resident children are not seen at KCH (18–20). Furthermore, challenges in childhood TB diagnosis, combined with limited diagnostic resources, make surveillance data from other health facilities unreliable. We therefore estimated the CDR of hospital-based surveillance at KCH by using 3 independent techniques. Each measure is necessarily a surrogate, and each has limitations, but the similarity of these estimates supports their validity.
Because we used the highest CDR estimate to generate conservative estimates of childhood TB incidence, the projected national incidence was 3 times higher than that reported. Nevertheless, the projected ratio of adult-to-child TB cases is still consistent with other studies in Africa (43,44) and with recent global estimates (1,5,6,9), although lower than some regional and global figures (3). Other estimates of the global TB burden have indicated a lower proportion of childhood cases (7,8). However, in the absence of data from children, those estimates assume a similar CDR for adults (8) or impute missing data based on reported proportions of smear-negative and extrapulmonary TB by age group (7), assumptions that have been challenged (11,45). Our study provides important empirical data on the probable CDR among children. The results suggest that the CDR among children is substantially lower than among adults and support estimates derived using other modeling approaches (5,6), including recently revised WHO estimates of global childhood TB incidence that assume a CDR of 36% (9).
The strong association of childhood TB with a history of close TB contact has 2 important implications for clinical practice and public health policy. First, eliciting a history of TB contact should be a standard part of the assessment of every ill child in TB-endemic settings. Among inpatients in our study, 1 in 5 with a known close TB contact had CHPTB. Early identification and investigation of this high-risk group might improve clinical outcomes through earlier diagnosis and treatment.
Second, and most important, our finding that 49% TB cases among children <5 years of age were attributable to a known household TB contact suggests that half the CHPTB cases in young children might have been prevented by chemoprophylaxis. Estimating the population attributable fraction of contact with a person with confirmed TB provides a novel approach for assessing the potential impact of TB chemoprophylaxis at the population level that might be applied to other settings. Our results from Kenya support recent global estimates of TB burden among child TB contacts (25). By demonstrating a large potential impact on childhood TB incidence, our findings provide further strong endorsement for existing policy recommendations for TB chemoprophylaxis (25,46).
Extrapolation of results from a single district must be interpreted with caution. Childhood TB incidence and the contribution of childhood TB cases to the total TB burden are likely to be affected by factors that vary geographically, including community TB prevalence; social and demographic factors, such as urbanization, that affect the annual risk for TB infection; prevalence of host factors, such as BCG vaccination, HIV infection, and malnutrition; and local population structures. Therefore, we did not attempt simply to age-standardize the Kilifi incidence rates to the national population of children in Kenya.
We reasoned instead that the proportion of the total TB caseload accounted for by children is probably less prone to geographic variation, and estimated the national burden of childhood TB by assuming that the CDR among adults and the ratio of adult-to-child cases is the same in the KHDSS and nationally. Importantly, the age structures of the KHDSS and Kenya are very similar (13,24), suggesting that age is unlikely to confound this approach. Compared with Kilifi, the higher estimate of TB incidence nationally is consistent with greater urbanization (13,24) and a higher annual risk for TB infection (47), HIV prevalence (24), and overall TB incidence (1). Because ecologic data suggest that the pediatric proportion of cases actually increases with increasing overall TB incidence (6,12), this approach might underestimate the actual national childhood TB burden. Our restriction of TB cases to those that met the stringent criteria of CHPTB and our adjustment of hospital-based incidence rates using the highest CDR estimate also suggest that our estimates are conservative.
In conclusion, by using a combination of clinical, laboratory, and epidemiologic resources not usually available for routine surveillance, we have estimated the incidence of childhood TB in Kenya. Although this study is very resource-intensive, the wide range of incidence estimates based on existing clinical definitions highlights the difficulty in interpreting routine notification data and reinforces the need for similar studies in a range of different epidemiologic settings. In a setting where routine facilities for childhood TB diagnosis are typical of most countries with a high TB burden, our results also provide important empirical data on the TB CDR among children. The results support recently improved WHO estimates of global childhood TB incidence based on modeling approaches, which assume a very similar CDR (1,9). Our findings also reinforce the urgent need to improve case detection among children to reduce childhood TB mortality (48). Crucially, they suggest that half the TB cases in young children might be prevented by implementing existing WHO guidelines for contact tracing and chemoprophylaxis.
Dr. Brent is a consultant and senior clinical lecturer in infectious diseases at the University of Oxford and Oxford University Hospitals NHS Foundation Trust. As a Wellcome Trust Fellow in tropical medicine, he worked at the KEMRI–Wellcome Trust Research Programme in Kilifi, Kenya, where the field work for this study was performed.
Acknowledgments
We would like to thank the clinical, laboratory, and administrative staff at Kilifi County Hospital and Coast Provincial General Hospital for their support of the study.
The study was supported by the Wellcome Trust in the form of research fellowships to A.J.B. (081697), T.N.W. (091758), and J.A.G.S. (098532) and a core grant to the KEMRI–Wellcome Trust Research Programme (077092). Additional funding for laboratory work was provided by a project grant from the Pneumonia Etiology Research for Child Health (PERCH) project funded by the Bill and Melinda Gates Foundation at Johns Hopkins Bloomberg School of Public Health. The funders had no role in data analysis or in the decision to publish.
Author contributions: A.J.B., J.A.G.S., and M.L. designed the study with input from C.R.J.N., T.N.W., C.N., E.B., J.S., and K.P. A.J.B., J.L., C.M., and J.W. recruited and followed up children with suspected TB. Chest radiographs were read and interpreted by A.J.B., J.S., and K.P. A.J.B. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- World Health Organization. Global tuberculosis report 2016. Geneva: The Organization; 2016.
- Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, et al.; for WHO’s Global TB Programme. WHO’s new end TB strategy. Lancet. 2015;385:1799–801. DOIPubMedGoogle Scholar
- Brent AJ. Childhood TB surveillance: bridging the knowledge gap to inform policy. J Trop Med. 2012;2012:865436. PMID: 22518169
- Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis. 2002;6:1038–45.PubMedGoogle Scholar
- Dodd PJ, Gardiner E, Coghlan R, Seddon JA. Burden of childhood tuberculosis in 22 high-burden countries: a mathematical modelling study. Lancet Glob Health. 2014;2:e453–9. DOIPubMedGoogle Scholar
- Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Pérez-Vélez CM, et al. Incidence of multidrug-resistant tuberculosis disease in children: systematic review and global estimates. Lancet. 2014;383:1572–9. DOIPubMedGoogle Scholar
- Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. DOIPubMedGoogle Scholar
- World Health Organization. Global tuberculosis report 2014. Geneva: The Organization; 2014.
- World Health Organization. Global tuberculosis report 2015. Geneva: The Organization; 2015.
- Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016;16:1193–201. DOIPubMedGoogle Scholar
- Seddon JA, Jenkins HE, Liu L, Cohen T, Black RE, Vos T, et al. Counting children with tuberculosis: why numbers matter. Int J Tuberc Lung Dis. 2015;19(Suppl 1):9–16. DOIGoogle Scholar
- Donald P, Maher D, Qazi S. A research agenda for childhood tuberculosis. Improving the management of childhood tuberculosis within national tuberculosis programmes: research priorities based on a literature review. Geneva: World Health Organization; 2007.
- Scott JA, Bauni E, Moisi JC, Ojal J, Gatakaa H, Nyundo C, et al. Profile: The Kilifi Health and Demographic Surveillance System (KHDSS). Int J Epidemiol. 2012;41:650–7. DOIPubMedGoogle Scholar
- World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second edition. Geneva: The Organization; 2014.
- Liebeschuetz S, Bamber S, Ewer K, Deeks J, Pathan AA, Lalvani A. Diagnosis of tuberculosis in South African children with a T-cell-based assay: a prospective cohort study. Lancet. 2004;364:2196–203. DOIPubMedGoogle Scholar
- Rachow A, Clowes P, Saathoff E, Mtafya B, Michael E, Ntinginya EN, et al. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: a prospective cohort study. Clin Infect Dis. 2012;54:1388–96. DOIPubMedGoogle Scholar
- Siddiqi SH, Ruesch-Gerdes S. MGIT procedure manual. Geneva: Foundation for Innovative New Diagnostics; 2006.
- Moïsi JC, Nokes DJ, Gatakaa H, Williams TN, Bauni E, Levine OS, et al. Sensitivity of hospital-based surveillance for severe disease: a geographic information system analysis of access to care in Kilifi district, Kenya. Bull World Health Organ. 2011;89:102–11. DOIPubMedGoogle Scholar
- Chuma J, Gilson L, Molyneux C. Treatment-seeking behaviour, cost burdens and coping strategies among rural and urban households in Coastal Kenya: an equity analysis. Trop Med Int Health. 2007;12:673–86. DOIPubMedGoogle Scholar
- Molyneux CS, Murira G, Masha J, Snow RW. Intra-household relations and treatment decision-making for childhood illness: a Kenyan case study. J Biosoc Sci. 2002;34:109–31.PubMedGoogle Scholar
- Bauni E, Ndila C, Mochamah G, Nyutu G, Matata L, Ondieki C, et al. Validating physician-certified verbal autopsy and probabilistic modeling (InterVA) approaches to verbal autopsy interpretation using hospital causes of adult deaths. Popul Health Metr. 2011;9:49. DOIPubMedGoogle Scholar
- Molyneux CS, Mung’Ala-Odera V, Harpham T, Snow RW. Maternal responses to childhood fevers: a comparison of rural and urban residents in coastal Kenya. Trop Med Int Health. 1999;4:836–45. DOIPubMedGoogle Scholar
- World Health Organization. Global tuberculosis control 2011: Kenya country profile. Geneva: The Organization; 2011.
- Kenya National Bureau of Statistics. Kenya Demographic and Health Survey 2008–09. Nairobi (Kenya): The Bureau; 2010.
- Yuen CM, Jenkins HE, Chang R, Mpunga J, Becerra MC. Two methods for setting child-focused tuberculosis care targets. Public Health Action. 2016;6:83–96. DOIPubMedGoogle Scholar
- Graham SM, Ahmed T, Amanullah F, Browning R, Cardenas V, Casenghi M, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205(Suppl 2):S199–208. DOIPubMedGoogle Scholar
- World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: The Organization; 2006.
- Stegen G, Jones K, Kaplan P. Criteria for guidance in the diagnosis of tuberculosis. Pediatrics. 1969;43:260–3.PubMedGoogle Scholar
- Nair PH, Philip E. A scoring system for the diagnosis of tuberculosis in children. Indian Pediatr. 1981;18:299–303.PubMedGoogle Scholar
- Hawkridge A, Hatherill M, Little F, Goetz MA, Barker L, Mahomed H, et al.; South African BCG trial team. Efficacy of percutaneous versus intradermal BCG in the prevention of tuberculosis in South African infants: randomised trial. BMJ. 2008;337(nov13 1):a2052.
- Stoltz AP, Donald PR, Strebel PM, Talent JMT. Criteria for the notification of childhood tuberculosis in a high-incidence area of the western Cape Province. S Afr Med J. 1990;77:385–6.PubMedGoogle Scholar
- Jeena PM, Mitha T, Bamber S, Wesley A, Coutsoudis A, Coovadia HM. Effects of the human immunodeficiency virus on tuberculosis in children. Tuber Lung Dis. 1996;77:437–43. DOIPubMedGoogle Scholar
- Edwards K. The diagnosis of childhood tuberculosis. P N G Med J. 1987;30:169–78.PubMedGoogle Scholar
- Ghidey Y, Habte D. Tuberculosis in childhood: an analysis of 412 cases. Ethiop Med J. 1983;21:161–7.PubMedGoogle Scholar
- World Health Organization. Provisional guidelines for the diagnosis and classification of the EPI target diseases for primary health care, surveillance and special studies. Geneva: The Organization; 1983.
- Ramachandran RS. Tuberculosis in children: experience with 1284 cases. Indian Pediatr. 1968;5:564–71.PubMedGoogle Scholar
- Osborne CM. The challenge of diagnosing childhood tuberculosis in a developing country. Arch Dis Child. 1995;72:369–74. DOIPubMedGoogle Scholar
- Fourie PB, Becker PJ, Festenstein F, Migliori GB, Alcaide J, Antunes M, et al. Procedures for developing a simple scoring method based on unsophisticated criteria for screening children for tuberculosis. Int J Tuberc Lung Dis. 1998;2:116–23.PubMedGoogle Scholar
- Cundall DB. The diagnosis of pulmonary tuberculosis in malnourished Kenyan children. Ann Trop Paediatr. 1986;6:249–55. DOIPubMedGoogle Scholar
- Kiwanuka J, Graham SM, Coulter JB, Gondwe JS, Chilewani N, Carty H, et al. Diagnosis of pulmonary tuberculosis in children in an HIV-endemic area, Malawi. Ann Trop Paediatr. 2001;21:5–14. DOIPubMedGoogle Scholar
- World Health Organization. Global tuberculosis control 2011. Geneva: The Organization; 2011.
- World Health Organization. Pocket book of hospital care for children: guidelines for the management of common illnesses with limited resources. Geneva: The Organization; 2005.
- Harries AD, Hargreaves NJ, Graham SM, Mwansambo C, Kazembe P, Broadhead RL, et al. Childhood tuberculosis in Malawi: nationwide case-finding and treatment outcomes. Int J Tuberc Lung Dis. 2002;6:424–31.PubMedGoogle Scholar
- Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Beyers N. The burden of childhood tuberculosis and the accuracy of community-based surveillance data. Int J Tuberc Lung Dis. 2006;10:259–63.PubMedGoogle Scholar
- Jenkins HE, Pagano M, Yuen CM, Becerra MC, Cohen T. The burden of tuberculosis disease in children—Authors’ reply. Lancet. 2014;384:1343–4. DOIPubMedGoogle Scholar
- Seddon JA. Knowing how many children to find is the first step in finding them. Public Health Action. 2016;6:52.PubMedGoogle Scholar
- Kwamanga D, Chakaya J, Sitienei J, Kalisvaart N, L’herminez R, van der Werf MJ. Tuberculosis transmission in Kenya: results of the third National Tuberculin Survey. Int J Tuberc Lung Dis. 2010;14:695–700.PubMedGoogle Scholar
- Starke JR. Mortality in childhood tuberculosis: has there been progress? Lancet Infect Dis. 2017;17:239–41. DOIPubMedGoogle Scholar
Figures
Tables
Cite This Article1The following members of the Kilifi Improving Diagnosis and Surveillance of Childhood TB (KIDS TB) Study Group also contributed to patient recruitment, investigation, and management: Victor Bandika, Jay Berkley, Kath Maitland, Susan Morpeth, Daisy Mugo, Robert Musyimi, Agnes Mutiso, John Paul Odhiambo, Monica Toroitich, and Hemed Twahir.
Prospective data on childhood tuberculosis (TB) incidence and case detection rates (CDRs) are scant, and the preventable burden of childhood TB has not been measured in prospective studies. We investigated 2,042 children (<15 years of age) with suspected TB by using enhanced surveillance and linked hospital, demographic, notification, and verbal autopsy data to estimate the incidence, CDR, risk factors, and preventable burden of TB among children in Kenya. Estimated TB incidence was 53 cases/100,000 children/year locally and 95 cases/100,000 children/year nationally. The estimated CDR was 0.20–0.35. Among children <5 years of age, 49% of cases were attributable to a known household contact with TB. This study provides much needed empiric data on TB CDRs in children to inform national and global incidence estimates. Moreover, our findings indicate that nearly half of TB cases in young children might be prevented by implementing existing guidelines for TB contact tracing and chemoprophylaxis.
Table of Contents – Volume 24, Number 3—March 2018
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Andrew J. Brent, Department of Microbiology, Level 6, John Radcliffe Hospital, Headington, Oxford OX3 9DU, UK
Top