Volume 25, Number 5—May 2019
Research
Serologic Prevalence of Ebola Virus in Equatorial Africa
Figure 4
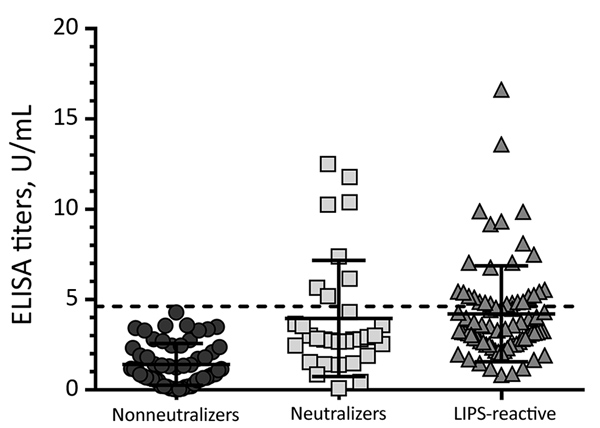
Figure 4. Summarized Ebola virus nucleoprotein ELISA data for confirmation of neutralizing and LIPS-reactive specimens across all sample sets in study of serologic prevalence of Ebola virus in equatorial Africa. For comparison, 57 random nonneutralizers were included. The ELISA cutoff value of 4.62 U/mL (dashed line) was determined on the basis of background reactivity for 47 serum samples from the local general population. Error bars indicate 95% CIs. LIPS, luciferase immunoprecipitation system.
1Current affiliation: University of Veterinary Medicine, Hannover, Germany.
Page created: April 17, 2019
Page updated: April 17, 2019
Page reviewed: April 17, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.