Volume 26, Number 12—December 2020
Research
Trends in Population Dynamics of Escherichia coli Sequence Type 131, Calgary, Alberta, Canada, 2006–20161
Abstract
Global expansion of antimicrobial drug–resistant Escherichia coli sequence type (ST) 131 is unrivaled among human bacteria. Understanding trends among ST131 clades will help with designing prevention strategies. We screened E. coli from blood samples (n = 1,784) obtained in Calgary, Alberta, Canada, during 2006, 2012, and 2016 by PCR for ST131 and positive samples (n = 344) underwent whole-genome sequencing. The incidence rate per 100,000 residents increased from 4.91 during 2006 to 12.35 during 2012 and 10.12 during 2016. ST131 belonged to clades A (10%), B (9%), and C (81%). Clades C1-nonM27 and B were common during 2006, and C2 containing blaCTX-M-15, C1-M27 containing blaCTX-M-27, and A were responsible for the increase of ST131 during 2012 and 2016. C2 was the most antimicrobial drug–resistant subclade and increased exponentially over time. Eradicating ST131, more specifically the C2 subclade, will lead to considerable public health benefits for persons in Calgary.
Escherichia coli sequence type (ST) 131 is the quintessential example of a successful, global, antimicrobial-resistant, high-risk clone among human bacteria (1,2). Currently, ST131 is the most common global extraintestinal pathogenic E. coli (ExPEC) clone; up to 30% of all ExPEC, 60%–90% of fluoroquinolone-resistant ExPEC, and 40%–80% of ExPEC with extended-spectrum β-lactamases [ESBLs] belong to ST131 (3,4). Population genetics indicate that ST131 consists of different clades (5): clade A contains serotype O16:H5 and fimH41, clade B contains mostly serotype O25b:H4 and fimH22, and clade C contains serotype O25b:H4 and fimH30. Clade C is divided into 2 subclades: C1/H30R (associated with fluoroquinolone resistance) and C2/H30Rx (associated with fluoroquinolone resistance and blaCTX-M-15). A novel ST131 C1 subclade, known as C1-M27 with blaCTX-M-27, was reported in Japan (6).
ST131 is the most dominant and most antimicrobial-resistant among E. coli causing bloodstream infections in Calgary, Alberta, Canada, infecting mostly the elderly in long-term care centers (7). Previous molecular epidemiology studies from the same region showed that ST131 was relatively rare among ESBL-producing and fluoroquinolone-resistant E. coli during the early 2000s but showed a major increase toward the end of the 2000s (8,9). However, limited information is available regarding the changes in population dynamics of ST131 clades over extended periods, especially among nonbiased E. coli isolates in large, well-defined, geographic regions.
To address this issue, we conducted a retrospective cohort study that characterized ST131 clades responsible for bloodstream infections in Calgary over an 11-year period (2006–2016). Investigating trends of ST131 clades over long periods by using a population-based surveillance approach will aid in clarifying the evolution of this clone and help with designing superior prevention strategies (3,10).
Study Population
We conducted a retrospective cohort study in Calgary by using all E. coli human clinical isolates from blood cultures processed by a centralized laboratory system (Alberta Precision Laboratories) during 2006, 2012, and 2016. All blood culture samples from adults and children in inpatient and outpatient settings were included.
Clinical Data
Clinical information corresponding to source patients at the time of the E. coli bloodstream infection was obtained by using Sunrise Clinical Manager (Allscripts Healthcare Solutions, Inc., https://www.allscripts.com). A case-patient with an E. coli bloodstream infection was defined as a patient with systemic inflammatory response and documented growth of an E. coli isolate in a blood culture. Incident case-patients were defined as Calgary residents with a first isolation of E. coli from blood. Repeat E. coli from blood were excluded. Bloodstream infections were defined as community acquired, hospital acquired, or healthcare associated (11).
Bacterial Isolates, Identification, and Susceptibility Testing
All E. coli isolates from blood were routinely stored at Alberta Precision Laboratories and available for this study. Unique isolates recovered during January 1–December 31, 2006, 2012, and 2016 were obtained from the frozen depository.
Identification was conducted by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Vitek; bioMérieux, https://www.biomerieux.com), and susceptibility testing was conducted the VITEK 2 Instrument (bioMérieux). Susceptibilities were determined for amoxicillin/clavulanic acid, piperacillin/tazobactam, ceftriaxone, meropenem, ertapenem, amikacin, gentamicin, tobramycin, ciprofloxacin, and trimethoprim/sulfamethoxazole. Throughout this study, results were interpreted by using the Clinical Laboratory Standards Institute criteria for broth dilution (12). Antimicrobial resistance and virulence scores were determined as described (13).
Molecular Characterization
All E. coli isolates (n = 1,786) were initially screened with a PCR specific for ST131 (14). Positive isolates (n = 344) underwent whole-genome sequencing, by using procedures previously (15,16). The Nextera XT DNA Sample Preparation Kit (Illumina, https://www.illumina.com) was used to prepare libraries for sequencing. Samples were multiplexed and sequenced on an Illumina NextSeq500 for 300 cycles (151-bp paired-end). Draft genomes were obtained by using SPAdes version 3.10.1 (17). To define the presence of genes and mutations, BLAST (18) in combination with following databases or typing schemes were accessed: National Center for Biotechnology Information Bacterial Antimicrobial Resistance Reference Gene Database (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047), ResFinder (19), PlasmidFinder (20), MLST (21) virulence finder (22), and virulence factor database (23). ST131 clades were identified by using an in silico PCR and primers described elsewhere (14).
Statistical Analysis
The Fisher exact test was used to perform pairwise comparisons of factors between clades, t-test was used for age comparisons, and p values obtained within individual categories were adjusted for multiple comparisons by using the false discovery rate (24). Population data were extracted from census reports from Statistics Canada (https://www.statcan.gc.ca) and used to estimate incidence rates (IRs) on the basis of a Poisson distribution. The Mann-Whitney test was used to compare antimicrobial resistance and virulence scores between clades. The effect of eliminating subclade C2 on nonsusceptibility and IRs was assessed by using Fisher exact and Poisson tests, for which population characteristics were compared with the presence and absence of subclade C2 isolates. The p values were adjusted for multiple comparisons accordingly. All analyses were conducted in R version 3.6.1 (25). Statistical significance was set at the 5% level.
Sequence Data Accession Numbers and Ethics
Sequencing data was deposited in the National Center for Biotechnology Information database (submission no. SUB7225977). This study was approved by the University of Calgary Conjoint Health Research Ethics Board (REB16-2457).
E. coli Isolates
E. coli was the most common bacterium obtained from blood in the Calgary region during 2006 (482 [28.9%] of 1,669 isolates), 2012 (691 [29.7%] of 2,084 isolates), and 2016 (685 [31.1%] of 2,201 isolates). A total of 1,786 unique E. coli were screened for ST131: 481 from 2006, 621 from 2012, and 684 from 2016. Overall, 344 (19.2%) of 1,786 E. coli isolates were PCR positive for ST131; the prevalence of ST131 increased from 53 (11%) of 481 during 2006 to 150 (24.2%) of 621 during 2012 and 141 (20.6%) of 684 during 2016 (p<0.001 for both comparisons).
Most ST131 isolates belonged to clade C in the following subclades (Table 1): C0 (n = 5, 2%), C1-nonM27 (n = 121, 35%), C1-M27 (n = 13, 4%), and C2 (n = 139, 40%). The remainder of ST131 isolates belonged to clades A (n = 34 [10%)] and B (n = 32 [9%]).
Incidence Rates and Population Dynamics of ST131 Clades
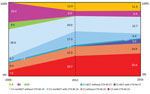
The IR per 100,000 residents with ST131 bloodstream infections in Calgary increased from 4.91 during 2006 to 12.35 during 2012 and 10.12 during 2016 (p<0.001 for both comparisons). Overall, the population structure of ST131 was dominated by the C clade. However, the IRs per 100,000 residents and proportions among the different subclades showed a major change over time (Table 2; Figure). The C0 subclade represented 9.4% of the ST131 population during 2006, with an estimated IR of 0.46 cases per 100,000 residents. However, the C0 subclade was not detected during 2012 and 2016 (p = 0.001 for both comparisons).
The C1-nonM27 subclade dominated the population structure of ST131 during 2006 (comprising of 46% of the total population, with an IR of 2.22/100,000 residents). Despite an increased IR during 2012 and 2016 (when compared with that for 2006), the frequency of C1-nonM27 isolates decreased to 37.3% in 2012 and 29% in 2016 (2006 vs. 2016; p = 0.04) (Figure). The C1-M27 subclade increased from 1.9% during 2006 to 5.7% during 2016. There was an association between C1-M27, the presence of blaCTX-M-27, and year of isolation (5 C1-M27 isolates from 2006 and 2012 were negative for blaCTX-M-27, and 7/8 isolates obtained during 2016 were positive for blaCTX-M-27) (p = 0.004) (Figure).
The prevalence of C2 subclade increased substantially from 17% of the total ST131 population during 2006 to 42% during 2012 and 47% during 2016 (p<0.001 for both comparisons) (Figure). The IR per 100,000 residents of the C2 clade increased from 0.83 during 2006 to 5.19 during 2012 and 4.81 during 2016 (p<0.001 for both comparisons) (Table 2). The increase in subclade C2 correlated with the presence of CTX-M-15 (4 [44%] of 9 of C2 isolates from 2006 were positive for blaCTX-M-15 compared with 89 [68%] of 130 isolates obtained during 2012 and 2016) (Figure).
Clade A was absent among ST131 during 2006 and then increased to 12% of the ST131 population during 2012 and 11.3% of the ST131 population during 2016 (p<0.01 for both comparisons) (Figure). The IR of clade A increased from 0 to 1.48/100,00 residents during 2012 and to 1.15/100,000, residents during 2016 (p<0.001 for both comparisons) (Table 2). B was the second most common clade during 2006 (26.4% of the total ST131 population), but decreased to 6% of the ST131 population during 2012 and to 6.4% of the ST131 population during 2016 (p<0.001 for both comparisons) (Figure). The IR of clade B decreased from 1.30/100,000 residents to 0.74/100,000 residents during 2012 and to 0.65/100,000 residents during 2016 (Table 2).
Clinical Characteristics
E. coli ST131 bloodstream infections were evenly distributed between male patients (n = 171, 49.7%) and female patients (n = 173, 50.3%) (Table 1). Just under half (48%) of E. coli ST131 bloodstream infections were healthcare-associated, followed by community-acquired (34%) and hospital-acquired (18%) (Table 1). Clades A and B were associated with community-acquired infections, and patients infected with clade C were more likely to be healthcare associated. Patients infected with clade A tended to be younger (Table 1). More than half (n = 186, 54%) of patients had upper urinary tract infections, followed by bloodstream infections with an unknown source (n = 69, 20%), pneumonia (n = 35, 10%), acute biliary tract infections (n = 31, 9%), and intraabdominal infections (n = 23, 7%) (Table 1).
Serotypes, fimH Types, and Antimicrobial Susceptibilities
Clade A contained O16:H5, fimH41, and fimH89. Clade B contained O25:H4, O2:H4, fimH22, fimH27, fimH324, and fimH30. Clade C contained O25:H4 and fimH30 (Table 1).
Overall, high (>25%), intermediate, or resistant (not susceptible) rates were observed for ceftriaxone, ciprofloxacin, trimethoprim/sulfamethoxazole, gentamicin, and tobramycin. Low rates (<5%) were observed for amikacin, ertapenem, and meropenem. C2 was the most antimicrobial-resistant subclade, followed by C1-nonM27 and C1-M27 (Table 1). Clades B and C0 were the most susceptible clades, and clade A showed high nonsusceptible rates for trimethoprim/sulfamethoxazole, gentamicin, and tobramycin (Table 1).
Removal of Subclade C2
Eliminating subclade C2 would have decreased the incidence rate of ST131 bloodstream infections from 12.35/100,000 residents to 7.16/100,000, residents during 2012 and from 10.12/100,00 residents to 5.31/100,000 residents during 2016 (p<0.001 both comparisons). In addition, eliminating subclade C2 would have resulted in a significant reduction of not susceptible rates for amoxicillin/clavulanic acid, ciprofloxacin, ceftriaxone, and tobramycin for ST131 causing bloodstream infections in Calgary (2006, 2012, and 2016) (p<0.05 for all comparisons).
Quinolone Resistance–Determining Regions and Antimicrobial Resistance Determinants
The combination of mutations in gyrase A genes (gyrA S83L and gyrA D87N) and DNA topoisomerase IV genes (parC S80I, parC E84V, and parE I529L) in the quinolone resistance-determining regions were present in all C1 and C2 isolates (Table 3). Nearly all (97%) ST131 isolates contained the parE I529L mutation. Most (85%) clade A isolates had the gyrA S83L mutation; for 5 isolates, this mutation was combined with gyrA D87N and parC S80I, and 1 isolate had the gyrA S83L, gyrA D87N, parC S80I, and parC E84V combination. Mutations in gyrA and parC were rare in clade B; 2/32 isolates had the gyrA S83L and parC S80I mutation combination (Table 3). One subclade C0 isolate had only the gyrA S83L mutation, and another C0 isolate had the gyrA S83L, gyrA D87N, parC S80I, and parC E84V combination.
CTX-M β-lactamases were detected among 148 (43%) isolates; most were CTX-M-15, followed by CTX-M-14, CTX-M-27, CTX-M-55, and CTX-M-198 (Table 3). CTX-M types were associated with different subclades (e.g., blaCTX-M-14 with C1-nonM27, blaCTX-M-15 with C2, blaCTX-M-27 with C1-M27, and blaCTX-M-55 with A). TEM-1 was common in most clades, with the exception of C2 and C1-M27. Three ST131 isolates were positive for blaCMY-2, and 1 C2 isolate was positive for blaNDM-5.
Certain aminoglycoside-modifying enzymes were common among ST131: aac(3)-IId, aac(6¢)-Ib-cr, aadA5, aph(3¢¢)-Ib, and aph (6)-Id (Table 3). Some associations between presences of aminoglycoside-modifying enzymes with certain subclades were noted: aac (3)-IId were present mainly in clades A, B, and C1-nonM27; aac(6')-Ib-cr in subclade C2; aadA2 in clade B, and aadA5 in clades A, C0, and C1. The combination of aph(3¢¢)-Ib and aph (6)-Id was more common in clades A and C1-nonM27 (Table 3). With regard to the presence of other antimicrobial resistance determinants, qnr was rare, and dfrA17, sul1, sul2, and tetA were common among most of ST131 clades (Table 3).
Plasmids and Replicon Types
Overall, IncF plasmid types (e.g., combinations of FIA, FIB, FIC, and FII) were common among all ST131 clades. Col-like plasmids and other plasmid families (IncI1, IncN, IncX1, IncX4, and IncY) were widely distributed across all clades but less common than IncF types (Table 1).
Using IncF plasmid replicons (FII_1, FIA_2, FIB_20, FII_2, and FIA_1) and a plasmid classification system published recently (26), we found that group 1 plasmids (combination of FII_1, FIA_2, and FIB_20) were in clades A, B, C1-nonM27, and C1-M27, and group 2 plasmids (combination of FII_2 and FIA_1) were in clades C0 and C2 (Table 1). Group 1 plasmids were common among C1 clades, and group 2 plasmids were common among C2 isolates.
Virulence-Associated Factors
The presence of 37 putative virulence factors were assessed for different clades (Table 4). The following factors were present among most isolates: papAIX, iha, fimH, sat, fyuA, usp, iss, and malX. Some virulence factors were associated with certain clades: papBCFJK, iha, hlyA, and cnf1 with subclade C2; afaABCD, draABCDP vat, and traT with clade A; afaABCD, draABCDP, kpsMII, and ibeABC with clade B; and kpsMTIII with subclades C0 and C1. No major differences in virulence scores were observed for the different clades.
The abrupt global expansion of ST131 during the 2000s is unrivaled among human bacteria and is a real-world model for the evolution of antimicrobial-resistant high-risk clones (10). This study describes the clinical features, incidence rates, genomic characteristics, and changes in population structure of ST131 clades causing bloodstream infections in a large centralized region of Canada over an 11-year period (2006–2016). The incidence rates and prevalence of ST131 increased over the time period, mostly caused by an influx of subclades C2 with blaCTX-M-15 and C1-M27 with blaCTX-M-27. Such results reinforce the possible role of CTX-M enzymes in the evolutionary success of ST131 (10). The presence of blaCTX-M-14 among C1-nonM27 isolates did not provide a beneficial advantage to this subclade. This finding is probably caused by clonal interference among 2 clones that have acquired different beneficial mutations competing in the same environment (27).
The population structure of ST131 in the Calgary region was dominated by clade C, which is similar to results from a previous large global study (28). The C clade originated from clade B during the mid to late 1980s by acquisition of several prophages, genomic islands, the fimH30 allele, and mutations within gyrA and parC that likely transpired in North America (29,30). The C clade in this study was mostly responsible for healthcare-associated urinary tract infections. C2 was the most common and most antimicrobial-resistant subclade in this collection and was associated with group 2 plasmids, blaCTX-M-15 and aac(6')-Ib-cr, as well as the virulence factors iha, hlyA, and cnf1. This subclade became prominent during 2012 and 2016 and showed the highest IRs among all subclades during this period. The increase of C2 correlated with the presence of CTX-M-15. Elimination of the C2 subclade through vaccination or phage-therapy programs (31), will lead to major decreases in incidence and antimicrobial-resistant rates among ST131 causing bloodstream infections in Calgary.
The C1-nonM27 subclade was the most common subclade during 2006 and associated with group 1 plasmids, blaCTX-M-14, and aac (3)-IId. Overall, the C1-M27 subclade was rare (especially during 2006 and 2012) but increased substantially during 2016, which correlated with the presence of blaCTX-M-27. The C1-M27 subclade has previously been responsible for increases in ESBL-producing E. coli from Japan and was also present among ST131 obtained from Thailand, Australia, Canada, and the United States (6). The ST131 C1-M27 subclade is currently emerging in Germany (32) and France (33) and is responsible for 27% of 144 clinical ST131 obtained from different sites in Europe (34).
Clade A is likely the ancestral lineage of ST131 and probably originated in Southeast Asia during the mid to late 1880s (30). Clade A isolates are generally sensitive to antimicrobial drugs and appear to occupy distinct ecologic niches, such as waste water (35). Results from this study show that clade A isolates have high not susceptible rates for trimethoprim/sulfamethoxazole, gentamicin, and tobramycin and were associated with community-associated and healthcare-associated urinary tract infections in younger patients. The virulence factors afaABCD, draABCDP, vat, and traT were common in clade A. Also, clade A was absent among ST131 from 2006 but became the third most common clade during 2012 and 2016, replacing clades B and C0 during these periods.
Clade B emerged from clade A in the early 1900s and most likely occurred in North America (10,30). Members of clade B are antimicrobial susceptible, and several intermediate subclades have been identified (29). Our study showed that clade B isolates were the second most common clade during 2006 but decreased substantially during 2016. This clade was the most antimicrobial sensitive ST131 clade in Calgary and was associated with community-acquired urinary tract infections and virulence factors afaABCD, draABCDP, kpsMII, and ibeABC.
Previous data have shown that gyrA S83L mutations occurred first among fluoroquinolone-resistant E. coli and is a major initial step for establishing relative fitness among antimicrobial-resistant isolates (36). Our study showed that gyrA mutations were rare among clade B isolates, but parE I529L mutations were common. This finding suggests that parE I529L mutations are the first to occur among fluoroquinolone-resistant ST131. The order in which these mutations arise might play a major role in establishing fitness in ST131 (37).
Our study had some limitations. Only patients in Calgary who had positive blood cultures for E. coli were included, which excluded those with E. coli bloodstream infections from whom no blood samples were submitted for culture. Therefore, incidence rates should be considered as conservative estimates of ST131 bloodstream infections in Calgary, especially for patients infected with clades A and B, who tended to be younger (i.e., clade A infections) and from the community (i.e., clade B infections). Such patients were less likely to have had blood cultures taken than patients who are older or who had previous contact with the healthcare system.
The novel approach for our study used population-based surveillance to describe the incidence rates, specific characteristics, and trends among ST131 clades over an 11-year period in a well-defined human population. We showed major differences in IRs, frequencies, resistance patterns, antimicrobial resistance determinants, grouped plasmid types, virulence factors, and trends over time for different clades. We provided insights into the evolution of ST131 clades in a large well-defined region of Canada. The population structure of ST131 in large geographic healthcare regions is dynamic and has continuous interplay between different subclades.
A previous study showed that eliminating ST131 would substantially decrease the overall IR and antimicrobial-resistant burden within E. coli causing bloodstream infections in the Calgary region (7). This study identified ST131 subclade C2 as the predominant and most antimicrobial-resistant subclade in Calgary, which is increasing exponentially over time. Eradicating ST131, more specifically the C2 subclade, will lead to considerable public health benefits for persons in Calgary.
Dr. Peirano is a research associate at Alberta Precision Laboratories and the University of Calgary, Calgary, Alberta, Canada. Her research interests include the molecular epidemiology of antimicrobial drug–resistant organisms.
Acknowledgment
This study was supported by research grant #10016015 from the Joint Programming Initiative on Antimicrobial Resistance/Canadian Institute Health Research Program.
References
- Mathers AJ, Peirano G, Pitout JD. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev. 2015;28:565–91. DOIGoogle Scholar
- Peirano G, Pitout JD. Molecular epidemiology of Escherichia coli producing CTX-M beta-lactamases: the worldwide emergence of clone ST131 O25:H4. Int J Antimicrob Agents. 2010;35:316–21. DOIGoogle Scholar
- Manges AR, Geum HM, Guo A, Edens TJ, Fibke CD, Pitout J. Global extraintestinal pathogenic Escherichia coli (ExPEC) lineages. Clin Microbiol Rev. 2019;32:e00135–18. DOIGoogle Scholar
- Peirano G, Pitout JDD. Extended-spectrum β-lactamase–producing Enterobacteriaceae: update on molecular epidemiology and treatment options. Drugs. 2019;79:1529–41. DOIGoogle Scholar
- Pitout JD, DeVinney R. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000 Res. 2017;6:6. DOIGoogle Scholar
- Matsumura Y, Pitout JD, Gomi R, Matsuda T, Noguchi T, Yamamoto M, et al. Global Escherichia coli sequence type 131 clade with blaCTX-M-27 gene. Emerg Infect Dis. 2016;22:1900–7. DOIGoogle Scholar
- Holland MS, Nobrega D, Peirano G, Naugler C, Church DL, Pitout JDD. Molecular epidemiology of Escherichia coli causing bloodstream infections in a centralized Canadian region: a population-based surveillance study. Clin Microbiol Infect. 2020;S1198-743X(20)30101-4.
- Peirano G, Pitout JD. Fluoroquinolone-resistant Escherichia coli sequence type 131 isolates causing bloodstream infections in a Canadian region with a centralized laboratory system: rapid emergence of the H30-Rx sublineage. Antimicrob Agents Chemother. 2014;58:2699–703. DOIGoogle Scholar
- Peirano G, van der Bij AK, Gregson DB, Pitout JD. Molecular epidemiology over an 11-year period (2000 to 2010) of extended-spectrum β-lactamase-producing Escherichia coli causing bacteremia in a centralized Canadian region. J Clin Microbiol. 2012;50:294–9. DOIGoogle Scholar
- Pitout JDD, Finn TJ. The evolutionary puzzle of Escherichia coli ST131. Infect Genet Evol. 2020;81:
104265 . DOIGoogle Scholar - Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. DOIGoogle Scholar
- Clinical and Laboratory Standards Institute. Perfomance standards for antimicrobial susceptibility testing. 25th information supplement. CLSI document M100–S25. Wayne (PA): The Institute; 2015.
- Johnson JR, Porter S, Thuras P, Castanheira M. The pandemic H30 subclone of sequence type 131 (ST131) as the leading cause of multidrug-resistant Escherichia coli infections in the United States (2011–2012). Open Forum Infect Dis. 2017;4:
ofx089 . DOIGoogle Scholar - Matsumura Y, Pitout JD, Peirano G, DeVinney R, Noguchi T, Yamamoto M, et al. RapidiIdentification of different Escherichia coli sequence type 131 clades. Antimicrob Agents Chemother. 2017;61:e00179–17. DOIGoogle Scholar
- Lowe M, Kock MM, Coetzee J, Hoosien E, Peirano G, Strydom KA, et al. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014–2016. Emerg Infect Dis. 2019;25:739–47. DOIGoogle Scholar
- Peirano G, Matsumura Y, Adams MD, Bradford P, Motyl M, Chen L, et al. Genomic epidemiology of global carbapenemase-producing Enterobacter spp., 2008–2014. Emerg Infect Dis. 2018;24:1010–9. DOIGoogle Scholar
- Nurk S, Bankevich A, Antipov D, Gurevich AA, Korobeynikov A, Lapidus A, et al. Assembling single-cell genomes and mini-metagenomes from chimeric MDA products. J Comput Biol. 2013;20:714–37. DOIGoogle Scholar
- Camacho C, Coulouris G, Avagyan V, Ma N, Papadopoulos J, Bealer K, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. DOIGoogle Scholar
- Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother. 2012;67:2640–4. DOIGoogle Scholar
- Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–903. DOIGoogle Scholar
- Zhou Z, Alikhan NF, Mohamed K, Fan Y, Achtman M; Agama Study Group. The EnteroBase user’s guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Res. 2020;30:138–52. DOIGoogle Scholar
- Joensen KG, Scheutz F, Lund O, Hasman H, Kaas RS, Nielsen EM, et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J Clin Microbiol. 2014;52:1501–10. DOIGoogle Scholar
- Liu B, Zheng D, Jin Q, Chen L, Yang J. VFDB 2019: a comparative pathogenomic platform with an interactive web interface. Nucleic Acids Res. 2019;47(D1):D687–92. DOIGoogle Scholar
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. DOIGoogle Scholar
- R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017.
- Kondratyeva K, Salmon-Divon M, Navon-Venezia S. Meta-analysis of pandemic Escherichia coli ST131 plasmidome proves restricted plasmid–clade associations. Sci Rep. 2020;10:36. DOIGoogle Scholar
- Hughes JM, Lohman BK, Deckert GE, Nichols EP, Settles M, Abdo Z, et al. The role of clonal interference in the evolutionary dynamics of plasmid–host adaptation. MBio. 2012;3:e00077–12. DOIGoogle Scholar
- Decano AG, Downing T. An Escherichia coli ST131 pangenome atlas reveals population structure and evolution across 4,071 isolates. Sci Rep. 2019;9:17394. DOIGoogle Scholar
- Ben Zakour NL, Alsheikh-Hussain AS, Ashcroft MM, Khanh Nhu NT, Roberts LW, Stanton-Cook M, et al. Sequential acquisition of virulence and fluoroquinolone resistance has shaped the evolution of Escherichia coli ST131. MBio. 2016;7:e00347–16.
- Stoesser N, Sheppard AE, Pankhurst L, De Maio N, Moore CE, Sebra R, et al.; Modernizing Medical Microbiology Informatics Group (MMMIG). Evolutionary history of the global emergence of the Escherichia coli epidemic clone ST131. MBio. 2016;7:
e02162 . DOIGoogle Scholar - Galtier M, De Sordi L, Maura D, Arachchi H, Volant S, Dillies MA, et al. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ Microbiol. 2016;18:2237–45. DOIGoogle Scholar
- Ghosh H, Doijad S, Falgenhauer L, Fritzenwanker M, Imirzalioglu C, Chakraborty T. blaCTX-M-27-encoding Escherichia coli sequence type 131 lineage C1-M27 clone in clinical isolates, Germany. Emerg Infect Dis. 2017;23:1754–6. DOIGoogle Scholar
- Birgy A, Bidet P, Levy C, Sobral E, Cohen R, Bonacorsi S. CTX-M-27-producing Escherichia coli of sequence type 131 and clade C1-M27, France. Emerg Infect Dis. 2017;23:885. DOIGoogle Scholar
- Merino I, Hernández-García M, Turrientes MC, Pérez-Viso B, López-Fresneña N, Diaz-Agero C, et al. R-GNOSIS Study Group. Emergence of ESBL-producing Escherichia coli ST131-C1-M27 clade colonizing patients in Europe. J Antimicrob Chemother. 2018;73:2973–80. DOIGoogle Scholar
- Finn TJ, Scriver L, Lam L, Duong M, Peirano G, Lynch T, et al. A comprehensive account of Escherichia coli sequence type 131 in wastewater reveals an abundance of fluoroquinolone-resistant clade A strains. Appl Environ Microbiol. 2020;86:e01913–9.
- Huseby DL, Pietsch F, Brandis G, Garoff L, Tegehall A, Hughes D. Mutation supply and relative fitness shape the genotypes of ciprofloxacin-resistant Escherichia coli. Mol Biol Evol. 2017;34:1029–39. DOIGoogle Scholar
- Johnson JR, Johnston B, Kuskowski MA, Sokurenko EV, Tchesnokova V. Intensity and mechanisms of fluoroquinolone resistance within the H30 and H30Rx subclones of Escherichia coli sequence type 131 compared with other fluoroquinolone-resistant E. coli. Antimicrob Agents Chemother. 2015;59:4471–80. DOIGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: November 09, 2020
1Accepted as an oral presentation for the 30th European Congress of Clinical Microbiology and Infectious Diseases, Paris, France, April 18–21, 2020.
2These authors contributed equally to this article.
Table of Contents – Volume 26, Number 12—December 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Johann D.D. Pitout, Department of Pathology and Laboratory Medicine, University of Calgary, #9 3535 Research Rd NW, Calgary T2L 2K8, Alberta, Canada
Top