Volume 27, Number 4—April 2021
Research
Emergence of Burkholderia pseudomallei Sequence Type 562, Northern Australia
Abstract
Since 2005, the range of Burkholderia pseudomallei sequence type 562 (ST562) has expanded in northern Australia. During 2005–2019, ST562 caused melioidosis in 61 humans and 3 animals. Cases initially occurred in suburbs surrounding a creek before spreading across urban Darwin, Australia and a nearby island community. In urban Darwin, ST562 caused 12% (53/440) of melioidosis cases, a proportion that increased during the study period. We analyzed 2 clusters of cases with epidemiologic links and used genomic analysis to identify previously unassociated cases. We found that ST562 isolates from Hainan Province, China, and Pingtung County, Taiwan, were distantly related to ST562 strains from Australia. Temporal genomic analysis suggested a single ST562 introduction into the Darwin region in ≈1988. The origin and transmission mode of ST562 into Australia remain uncertain.
The tropical disease melioidosis causes sepsis in persons with risk factors such as diabetes or hazardous alcohol consumption (1). The causative bacterium, Burkholderia pseudomallei, is found in soil and surface waters. Most reported cases of melioidosis occur in Southeast Asia and northern Australia during the monsoonal wet seasons (i.e., November–April in northern Australia and May–October in Southeast Asia) (1). Although melioidosis is increasingly found in China, the Pacific Islands, South Asia, Africa, and Central and South America (2), laboratory diagnostic constraints contribute to underreporting of cases. As a result, the true global distribution and prevalence of melioidosis remain uncertain (3).
Clinical manifestations are varied; however, pneumonia is the most common form, accounting for ≈50% of cases (4). The frequencies of these manifestations differ by region. For example, suppurative parotitis is common in children in Thailand and Cambodia but rare in Australia; manifestations such as prostate abscesses and brainstem encephalitis are reported rarely outside Australia (1,4). Death rates range from 9% in northern Australia to 40% in northeast Thailand (1,4). The extent to which transmission mode, host risk factors, access to diagnostic testing, appropriate antimicrobial drugs, and intensive care treatment account for differences in manifestations and outcomes remains uncertain. Clinical studies suggest that host risk factors are major contributors to disease severity and outcome (1).
Phylogeographic analyses suggest that B. pseudomallei emerged in ancient Australia and subsequently disseminated throughout Asia (2,5,6). Because of their ecologic niche, sensitivity to ultraviolet light, and rare transmission among humans, strains of B. pseudomallei in Australia have remained phylogenetically distinct from strains in Asia, Africa, and the Americas (2,6). Most reported instances of sequence type (ST) overlap between Asia and Australia are unrelated at the whole-genome level (7), with the exception of ST562 (8). Some STs in Southeast Asia occur over large geographic areas, such as along the Mekong and other rivers where erosion and washout from disturbed land might have contributed to B. pseudomallei dissemination (2,9,10).
Within Australia, most B. pseudomallei STs have a restricted geographic range (11). In the urban and rural areas of Darwin, STs have been found in the environment distributed across no more than 50 km (12). In Northern Australia, researchers have identified only 2 instances of long-range B. pseudomallei dispersal, spanning distances of 90 km and 460 km (13,14). B. pseudomallei isolates in the Northern Territory of Australia are very diverse, belonging to at least 379 reported STs (12). In this region, strains found in clinical and environmental samples exhibit similar levels of diversity (11). High species diversity of B. pseudomallei exists in urban Darwin; however, several STs, including ST109, ST36, ST132, and in recent years, ST553, have dominated among clinical and environmental isolates (7,15).
The Darwin Prospective Melioidosis Study has documented every culture-confirmed melioidosis case in the Top End region of the Northern Territory since 1989. In 2005, we reported the emergence of B. pseudomallei ST562 in urban Darwin (8). Genomic analyses revealed limited diversity among isolates and a very narrow geographic range, suggesting a single, recent introduction event from Asia (8). We describe the clinical manifestations and genomic epidemiology of B. pseudomallei ST562, which is now well-established in urban Darwin and causes a large proportion of melioidosis cases in the region.
Melioidosis Cases
We conducted this study at Royal Darwin Hospital, the referral center for the Top End region. The Top End is in the wet-dry tropics, ≈245,000 km2 in area, and sparsely populated. Darwin, the only city in the region, has a population of ≈122,000 persons; the remaining population lives in towns or remote communities separated by vast geographic distances. As part of the Darwin Prospective Melioidosis Study, we documented the demographic characteristics, risk factors, clinical features, and outcomes of 1,148 patients with culture-confirmed melioidosis during October 1, 1989–September 30, 2019 (1). We conducted multilocus sequence typing on isolates from 1,108 of 1,148 patients (https://pubmlst.org/organisms/burkholderia-pseudomallei) (16). Our study was approved by the Human Research Ethics Committee of the Northern Territory Department of Health and the Menzies School of Health Research, Darwin.
B. pseudomallei Isolates
We analyzed B. pseudomallei ST562 sequences from 61 humans (including 3 with recurrent infection), 3 animals, and 4 environmental samples in the Top End; 5 isolates from humans in Hainan Province, China; and 1 isolate from a human in Pingtung County, Taiwan. We conducted whole-genome sequencing using HiSeq 2000, HiSeq 2500, HiSeq 3000, MiSeq, or NovaSeq 6000 (Illumina, Inc., https://www.illumina.com). We also analyzed the genome of a ST562 strain isolated from a water source in Haikou city, Hainan Province, in 1975 (17). In addition, we conducted a global phylogenetic analysis using 281 non-ST562 B. pseudomallei genomes available in public sources (Appendix 1 Table). We displayed the geographic distribution of Australia ST562 isolates using ArcGIS (https://www.arcgis.com/index.html) with shape files provided by the government of the Northern Territory.
Statistical Analyses
We conducted all analyses using R version 3.6.0 (http://www.r-project.org). We used a 2-tailed Fisher exact test to conduct a bivariate analysis of demographic characteristics, underlying conditions, clinical features, and outcomes of 53 patients with ST562 and 387 patients with non-ST562 B. pseudomallei infection during October 1, 2004–September 30, 2019 in urban Darwin. We considered significant characteristics (i.e., p<0.05 in bivariate analysis) in a binomial multivariable generalized linear model with ST562 infection as the outcome. Because of the strong temporal structure to the data, we also included year of diagnosis as a continuous variable (Appendix 2).
Bioinformatic Analyses
We conducted multiple sequence alignment and variant calling with Snippy version 4.3.6 (https://github.com/tseemann/snippy), using the closed ST562 MSHR5858 genome (18) (GenBank accession nos. CP008891–2) as the reference for ST562 phylogenetic analyses and the closed K96243 genome (19) (GenBank accession nos. BX571965–6) for the global analysis. We conducted maximum-likelihood phylogenetic analyses using IQ-TREE version 1.6.10 (20) and predicted regions of recombination using Gubbins version 2.3.4 (21). We used BEAST 2 (22) for temporal analysis of the core Australia ST562 alignment (Appendix 2).
Australia B. pseudomallei ST562 Epidemiology
During 1989–2019, a total of 61 (5.5%) of 1,108 melioidosis cases were caused by B. pseudomallei ST562. After treatment completion, 3 (5%) patients had recurrent ST562 infection. Fifty-three (87%) ST562 patients resided in urban Darwin and 5 (8%) in an island community 81 km north of Darwin. In addition, 1 patient was evacuated from a remote community in East Arnhem Land, Northern Territory, Australia, 6 days after returning from a visit in Darwin; 1 patient lived in a rural community 37 km from Darwin; and 1 patient with an unknown travel history sought treatment at Katherine District Hospital (Katherine, Northern Territory, Australia), 317 km south of Darwin.
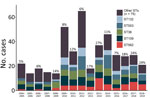
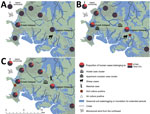
During 2005–2019, the proportion of human melioidosis cases caused by ST562 in urban Darwin gradually increased (Figure 1). These cases mostly occurred in 2 hotspot regions: suburbs surrounding a creek where 17 (30%) of 57 melioidosis cases were caused by ST562 and a lagoon where 11 (38%) of 29 cases were caused by ST562. The geographic distribution of cases changed over the 15-year period, moving initially from the creek hotspot to other regions in Darwin and to the island community (Figure 2). Records showed 2 case clusters with known epidemiologic links; the first cluster comprised 5 patients at a hostel in the lagoon hotspot during January 2014–March 2019 and the second comprised 2 persons from separate apartments in the same complex who were each found dead in their apartments on the same day in January 2014. B. pseudomallei ST562 was isolated from the autopsy samples of the 2 persons.
In addition, ST562 infections developed in 2 sheep at a veterinary facility in 2009 and 2014 (8) and in a meerkat at a wildlife park in 2015 (23). These cases occurred in the lagoon hotspot. Environmental sampling at these facilities did not reveal B. pseudomallei ST562. Furthermore, despite extensive systematic sampling across Darwin in 2017–2018, researchers found ST562 on only 2 occasions, both at the creek hotspot (15). During the investigation of a 2011 melioidosis case in a human, we isolated ST562 from air and soil samples from the lagoon hotspot (24).
ST562 Risk Factors and Clinical Features
Among 440 melioidosis patients in urban Darwin during October 1, 2004–September 30, 2019, a significantly higher proportion of patients with ST562 were Aboriginal or Torres Strait Islander (66% vs. 44%; p<0.01), lived in the suburbs surrounding the creek hotspot (33% vs. 11%; p<0.01) or the lagoon hotspot (21% vs. 5%; p<0.01), or reported hazardous alcohol consumption (59% vs. 40%; p = 0.02) (Table 1). In a generalized linear model that included these predictors, only residence in either of the 2 hotspot locations was a significant predictor of infection (Table 1). Pneumonia was the most common manifestation among patients with ST562 (76%) and non-ST562 infections (68%) (Table 2). Among male patients, 10 (32%) with ST562 had a prostate abscess, compared with 33 (15%; p = 0.02) men with non-ST562 infections. In total, 3 patients with ST562 infection died before hospitalization. The proportion of patients with bacteremia, septic shock, or death from melioidosis was not different among those with ST562 versus non-ST562 infection (Table 2).
Australian B. pseudomallei ST562 Diversity
The 71 ST562 isolates from Australia were closely related with 141 single-nucleotide polymorphisms (SNPs) over a core alignment length of 7,071,987 nucleotides. These isolates were substantially less diverse than isolates of ST109, ST36, and ST132, which also are found in Darwin (8). The median pairwise difference among ST562 genomes was 5 SNPs (range 0–16 SNPs). We found a limited phylogenetic structure among the ST562 genomes, with multiple polytomies on maximum-likelihood analysis (Figure 3); we did not identify any recombination. The soil isolate collected from the lagoon hotspot, MSHR4681, differed by only 5 SNPs from the first soil isolate from the creek hotspot, MSHR10541, despite being collected 6 years and 12 km apart. Isolates from 3 patients (MSHR8799, MSHR9707, and MSHR11750) had no known epidemiologic links but were separated by 0 SNPs.
B. pseudomallei ST562 Genomic Clusters
The ST562 isolates from cases associated with the hostel were phylogenetically clustered (Figure 3). The first 2 cases occurred 1 day apart in January 2014 and the third occurred in March 2014; isolates from these 3 cases differed by 0 SNPs. A fourth case in December 2014 differed from the first 3 isolates by 1 SNP and a fifth case in March 2019 differed by an additional SNP. This clade also included an ST562 isolate from a patient not initially known to have resided at the hostel; further investigation revealed that this patient had checked out of the hostel 6 days before being evacuated from a remote community for melioidosis treatment. Environmental sampling at the hostel did not detect ST562. We advised hostel staff regarding melioidosis prevention, including the importance of protective footwear and remaining indoors during storms.
The isolates from the 2 deceased persons from the apartment complex differed by 7 SNPs and did not fall within the same clade on the phylogenetic tree (Figure 3). However, an isolate from 1 of these patients was separated by 0 SNPs from a clinical isolate collected 10 months earlier from a patient who lived in the adjacent unit. Soil and water sampling of the apartment complex and its surroundings did not identify any B. pseudomallei ST562 isolates.
ST562 isolates from air and soil samples at a residence in the lagoon hotspot were separated by 0 SNPs. These isolates differed by 3 SNPs from a clinical isolate from a resident with bacteremic mediastinal melioidosis (24). Thirteen other clinical isolates were more closely related to the air and soil isolates than the clinical isolate from the resident; 1 isolate from a patient 8 years later was identical to the soil and air isolates. That patient lived 3.4 km downwind from the environmental sampling site.
Recurrent B. pseudomallei ST562 Infections
Of the 3 recurrent ST562 cases, genomic analysis confirmed that 1 case was a new infection and 2 were relapses (Figure 3). The first patient was treated for melioidosis pneumonia in January 2009 and March 2016; isolates from these 2 episodes differed by 9 SNPs and belonged to different phylogenetic clades, suggesting that these illnesses were caused by independent infection events (25). The second patient had B. pseudomallei isolated from urine in December 2017. This patient was treated with intravenous therapy for 4 weeks with ceftazidime then meropenem, then for 12 weeks with oral doxycycline. In October 2018, B. pseudomallei was again isolated from this patient’s urine; the 2 isolates differed by 5 SNPs and belonged to the same clade, suggesting relapse. The third patient had acute pneumonia in January 2019 and was treated for 4 weeks with intravenous meropenem then ceftazidime, then for 12 weeks with oral trimethoprim/sulfamethoxazole. His symptoms improved and he returned to his community but was subsequently found dead in August 2019. His autopsy revealed pneumonia caused by an isolate differing from his original infection by only 1 SNP.
B. pseudomallei ST562 Origin and Dispersal
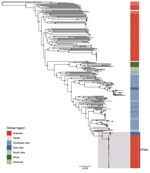
The mean estimated clock rate for the 71 Australia B. pseudomallei ST562 isolates was 4.11 × 10–8 substitutions/site/year (95% highest posterior density [HPD] 2.0–6.2 × 10–8 substitutions/site/year) and the median estimate for the time to the most recent common ancestor was 1988 (95% HPD 1961–2001) (Figure 4). Isolates from the creek hotspot predominated on the deepest branching clades and were distributed throughout the phylogeny, indicating initial establishment in and dispersal from the creek hotspot. Isolates from patients from the island community formed a clade estimated to have diverged from a common ancestor in 2010 (95% HPD 2004–2014). The 5 isolates from Hainan and the isolate from Taiwan were not included in the molecular dating analysis due to poor clock signal; these isolates were distantly related to ST562 isolates from Australia, differing by 6,252–7,786 SNPs (964–1,453 SNPs when excluding recombinogenic regions). In the global B. pseudomallei analysis, B. pseudomallei ST562 isolates from Australia were most closely related to isolates from East Asia (Figure 5). The ST562 clade belonged to the larger Asian clade in the global phylogeny (8).
B. pseudomallei ST562 emerged in northern Australia in 2005, fifteen years after the Darwin Prospective Melioidosis Study began genomic surveillance (1,8). Initially, cases of ST562 in northern Australia mostly occurred in a creek hotspot before spreading across Darwin and to an island community to the north. A La Niña period of heavy rainfall during 2010–2012 was associated with increased melioidosis case numbers in Darwin (26). After this period, the geographic distribution and proportion of cases attributable to ST562 rose. Increased connectivity of waterways and wet conditions might have contributed to ST562 spread in Darwin during this time.
The clinical manifestations, symptom duration, and severity of melioidosis caused by ST562 were similar to those caused by non-ST562 infections, suggesting that host risk factors and route of acquisition contributed to clinical features more than differences in virulence profiles (1,27). The only difference in clinical manifestations was a larger proportion of male ST562 patients with prostate abscesses. Compared with the rate in Asia, the greater melioidosis survival rates observed in Australia are probably improved by greater access to treatment, including intensive care (1,4).
Genomic analysis of ST562 strains from Australia demonstrated very little diversity, suggesting a single introduction event with a probable origin in Asia (8). The only other characterized ST562 isolates in this study are from Hainan Province, China, and from Pingtung County, Taiwan. Comparative genomic analysis showed that the strains from China and Taiwan strains belonged the same clade but were distantly related to strains in Australia. Researchers have not identified any close relatives of ST562 strains in Australia; their precise origin within Asia remains uncertain.
We estimated that the most recent common ancestor of the ST562 strains in Australia, which indicates the possible time of introduction, occurred in 1988; however, the 95% HPD for this estimate was wide (1961–2001). The estimated clock rate of 4.11 × 10–8 substitutions/site/year was lower than previously reported. For example, previous reports estimated the rate for serial isolates in patients with cystic fibrosis as 4.9 × 10–7 substitutions/site/year (28) and for isolates from a 16-year chronic lung infection as 1.7 × 10–7 substitutions/site/year (29). For B. pseudomallei groups in Asia and the Americas, the estimated mutation rates range from 1.12 × 10–6 to 9.22 × 10–7 substitutions/site/year (2). The variation in these estimates might reflect the difficulty in identifying and excluding SNPs resulting from recombination, the different ecologic conditions and selective pressures of isolates, and inadequate sampling.
B. pseudomallei can exist in a viable but nonculturable state (30) and can persist in the environment in suboptimal conditions outside of regions to which it is endemic; the slow replication rate during these periods might contribute to its slow accumulation of mutations. Cases have sporadically occurred in temperate Western Australia, where 2 isolates from animals on different farms collected 17 years apart differed by just 1 SNP (31). In contrast, the bacteria can evolve rapidly during acute infection; in 1 patient, 8 SNPs and 5 small insertions/deletions developed in a 12-day period (32). We observed similar variability; for example, environmental and clinical samples collected 8 years apart differed by 0 SNPs, whereas isolates collected 10 months apart from the same patient differed by 5 SNPs. B. pseudomallei replication is probably greater in vivo, with the human host milieu placing the bacterium under greater selective pressure than the natural environment.
Previous epidemiologic investigations of melioidosis clusters in humans and animals suggest differences of <1 SNP from an implicated infecting source (15,23,33,34). In the investigation of 2 cases of infection with B. pseudomallei ST325 on the same rural property in northern Australian, there was 1 SNP difference between the 2 clinical isolates and the suspected environmental source, an unchlorinated water tank (33). In a fatal outbreak in a remote island community in northern Australia, 4 B. pseudomallei ST126 clinical isolates differed by <1 SNPs from an isolate from the town water supply (34). We confirmed case clusters at a hostel and an apartment complex by combining epidemiologic information and phylogenetic analysis, enabling the identification of previously unassociated cases. There was little diversity among B. pseudomallei ST562 isolates in northern Australia; many epidemiologically unrelated isolates differed by ≤1 SNP. Phylogenetic analysis was required for cluster identification.
Intercontinental dispersal of B. pseudomallei is an extremely unusual event, as demonstrated by the strong phylogeographic signal in the global phylogeny (2,5–8). The mode of ST562 transmission into northern Australia and then to an offshore island is unclear but could have been through soil, plants, animals, or humans (8) or through air during a severe weather event. The bacterium does not survive prolonged exposure to ultraviolet light, which probably limits aerial dispersal (35); many researchers consider long-range intercontinental spread through the air unlikely. However, B. pseudomallei has been isolated from air samples (24,36), suggesting that this route of transmission might be possible across relatively short distances. The dissemination of B. pseudomallei across islands in the Caribbean might have been mediated by hurricanes (37), and the dispersal of B. pseudomallei across northern Australia might be associated with tropical cyclones (38). Tropical cyclones occur every year in northern Australia, and melioidosis clusters can occur after these events (31,39). Clusters also have been associated with typhoons in Taiwan, where studies using multilocus sequence typing have suggested airborne dissemination from soil and an increase in human cases depending on the wind direction (36,40). The distance that B. pseudomallei can travel in such events remains uncertain.
Although B. pseudomallei colonization of humans is rare (29,41), the bacterium has been found in the feces of domestic and wild animals including wallabies, horses, and chickens (42,43), and in the beak of a healthy native peaceful dove (Geopelia placida) (44). A strong association exists between B. pseudomallei presence in soil and disturbance by horses, chickens, and pigs (45). B. pseudomallei has been imported into areas to which it is not endemic and that are associated with exotic animals (46,47), the most dramatic example of which was an outbreak in a zoo in Paris that caused the deaths of >2 humans and animals belonging to >10 different species (43,48). The outbreak spread from the Paris Zoological Park to the menagerie at the Paris Botanical Gardens and equestrian clubs across France. B. pseudomallei was isolated from horse manure in multiple gardens and from petri dishes placed near manure, suggesting aerosolization. Movement of horses for races contributed to the outbreak.
Animal importation and migratory birds are possible modes by which ST562 could have arrived in Darwin. In the lagoon hotspot, 2 facilities that housed imported animals were the sites of 3 cases in animals of melioidosis caused by B. pseudomallei ST562. A horse racetrack and multiple equestrian clubs are also in the creek and lagoon hotspots. Both hotspots are habitats for water birds, many of which migrate to the region every year through Asia’s great flyways (49) and which could have carried B. pseudomallei ST562 to Darwin from Asia.
Although phylogenetic analysis confirms a single introduction event of Asian origin, how ST562 spread into northern Australia remains unknown. ST562 is now one of the most common B. pseudomallei STs in humans in urban Darwin. However, this ST rarely is isolated from the environment, including at sites associated with outbreaks. Further focused environmental sampling at key sites will help clarify ST562 epidemiology in northern Australia. The expanding capacity for genomic sequencing of B. pseudomallei will probably increase awareness of the ongoing global and regional dispersal of this bacterium and consequent melioidosis cases in humans and animals.
Dr. Meumann is an infectious diseases physician at Royal Darwin Hospital, Darwin. Her research interests include genomic epidemiology of infectious diseases in northern Australia.
Acknowledgments
We thank Glenda Harrington and Vanessa Rigas for field sampling and laboratory support and Derek Sarovich for Burkholderia pseudomallei genome curation. We are grateful to Rob Baird and staff at Territory Pathology, Darwin, for clinical isolates and to Suresh Benedict and staff at Berrimah Veterinary Laboratory, Darwin, for animal isolates. We also thank Xiao Zheng, Ya-Lei Chen, and Jung-Jung Mu for sharing B. pseudomallei sequence type 562 isolates and genomes.
This research was funded by the Australian National Health and Medical Research Council (grant nos. 1046812, 1098337, and 1131932 [The HOT NORTH initiative]). E.M.M. was funded by a postgraduate scholarship from the Australian National Health and Medical Research Council (grant no. 1114696). E.P.P. was funded by an Advance Queensland fellowship (grant no. AQIRF0362018).
References
- Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:
e900 . DOIPubMedGoogle Scholar - Chewapreecha C, Holden MT, Vehkala M, Välimäki N, Yang Z, Harris SR, et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat Microbiol. 2017;2:16263. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. DOIGoogle Scholar
- Wiersinga WJ, Currie BJ, Peacock SJ. Melioidosis. N Engl J Med. 2012;367:1035–44. DOIPubMedGoogle Scholar
- Pearson T, Giffard P, Beckstrom-Sternberg S, Auerbach R, Hornstra H, Tuanyok A, et al. Phylogeographic reconstruction of a bacterial species with high levels of lateral gene transfer. BMC Biol. 2009;7:78. DOIPubMedGoogle Scholar
- Sarovich DS, Garin B, De Smet B, Kaestli M, Mayo M, Vandamme P, et al. Phylogenomic analysis reveals an Asian origin for African Burkholderia pseudomallei and further supports melioidosis endemicity in Africa. MSphere. 2016;1:e00089–15. DOIPubMedGoogle Scholar
- De Smet B, Sarovich DS, Price EP, Mayo M, Theobald V, Kham C, et al. Whole-genome sequencing confirms that Burkholderia pseudomallei multilocus sequence types common to both Cambodia and Australia are due to homoplasy. J Clin Microbiol. 2015;53:323–6. DOIPubMedGoogle Scholar
- Price EP, Sarovich DS, Smith EJ, MacHunter B, Harrington G, Theobald V, et al. Unprecedented melioidosis cases in Northern Australia caused by an Asian Burkholderia pseudomallei strain identified by using large-scale comparative genomics. Appl Environ Microbiol. 2015;82:954–63. DOIPubMedGoogle Scholar
- Zimmermann RE, Ribolzi O, Pierret A, Rattanavong S, Robinson MT, Newton PN, et al. Rivers as carriers and potential sentinels for Burkholderia pseudomallei in Laos. Sci Rep. 2018;8:8674. DOIPubMedGoogle Scholar
- Ribolzi O, Rochelle-Newall E, Dittrich S, Auda Y, Newton PN, Rattanavong S, et al. Land use and soil type determine the presence of the pathogen Burkholderia pseudomallei in tropical rivers. Environ Sci Pollut Res Int. 2016;23:7828–39. DOIPubMedGoogle Scholar
- McRobb E, Kaestli M, Price EP, Sarovich DS, Mayo M, Warner J, et al. Distribution of Burkholderia pseudomallei in northern Australia, a land of diversity. Appl Environ Microbiol. 2014;80:3463–8. DOIPubMedGoogle Scholar
- Chapple SNJ, Price EP, Sarovich DS, McRobb E, Mayo M, Kaestli M, et al. Burkholderia pseudomallei genotype distribution in the Northern Territory, Australia. Am J Trop Med Hyg. 2016;94:68–72. DOIPubMedGoogle Scholar
- Aziz A, Sarovich DS, Harris TM, Kaestli M, McRobb E, Mayo M, et al. Suspected cases of intracontinental Burkholderia pseudomallei sequence type homoplasy resolved using whole-genome sequencing. Microb Genom. 2017;3:3. DOIPubMedGoogle Scholar
- Rachlin A, Kleinecke M, Kaestli M, Mayo M, Webb JR, Rigas V, et al. A cluster of melioidosis infections in hatchling saltwater crocodiles (Crocodylus porosus) resolved using genome-wide comparison of a common north Australian strain of Burkholderia pseudomallei. Microb Genom. 2019;5:5. DOIPubMedGoogle Scholar
- Rachlin A, Mayo M, Webb JR, Kleinecke M, Rigas V, Harrington G, et al. Whole-genome sequencing of Burkholderia pseudomallei from an urban melioidosis hot spot reveals a fine-scale population structure and localised spatial clustering in the environment. Sci Rep. 2020;10:5443. DOIPubMedGoogle Scholar
- Godoy D, Randle G, Simpson AJ, Aanensen DM, Pitt TL, Kinoshita R, et al. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J Clin Microbiol. 2003;41:2068–79. DOIPubMedGoogle Scholar
- Song L, Yu Y, Feng L, He J, Wang T, Zhu H, et al. Draft genome sequence of Burkholderia pseudomallei strain 350105, isolated in Hainan, China, in 1976. Genome Announc. 2015;3:e01162–15. DOIPubMedGoogle Scholar
- Daligault HE, Davenport KW, Minogue TD, Bishop-Lilly KA, Broomall SM, Bruce DC, et al. Whole-genome assemblies of 56 burkholderia species. Genome Announc. 2014;2:2.PubMedGoogle Scholar
- Holden MT, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101:14240–5. DOIPubMedGoogle Scholar
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32:268–74. DOIPubMedGoogle Scholar
- Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:
e15 . DOIPubMedGoogle Scholar - Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu CH, Xie D, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2014;10:
e1003537 . DOIPubMedGoogle Scholar - Rachlin A, Shilton C, Webb JR, Mayo M, Kaestli M, Kleinecke M, et al. Melioidosis fatalities in captive slender-tailed meerkats (Suricata suricatta): combining epidemiology, pathology and whole-genome sequencing supports variable mechanisms of transmission with one health implications. BMC Vet Res. 2019;15:458. DOIPubMedGoogle Scholar
- Currie BJ, Price EP, Mayo M, Kaestli M, Theobald V, Harrington I, et al. Use of whole-genome sequencing to link Burkholderia pseudomallei from air sampling to mediastinal melioidosis, Australia. Emerg Infect Dis. 2015;21:2052–4. DOIPubMedGoogle Scholar
- Sarovich DS, Ward L, Price EP, Mayo M, Pitman MC, Baird RW, et al. Recurrent melioidosis in the Darwin Prospective Melioidosis Study: improving therapies mean that relapse cases are now rare. J Clin Microbiol. 2014;52:650–3. DOIPubMedGoogle Scholar
- Parameswaran U, Baird RW, Ward LM, Currie BJ. Melioidosis at Royal Darwin Hospital in the big 2009-2010 wet season: comparison with the preceding 20 years. Med J Aust. 2012;196:345–8. DOIPubMedGoogle Scholar
- Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–25. DOIPubMedGoogle Scholar
- Viberg LT, Sarovich DS, Kidd TJ, Geake JB, Bell SC, Currie BJ, et al. Within-host evolution of Burkholderia pseudomallei during chronic infection of seven Australasian cystic fibrosis patients. MBio. 2017;8:e00356–17. DOIPubMedGoogle Scholar
- Pearson T, Sahl JW, Hepp CM, Handady K, Hornstra H, Vazquez AJ, et al. Pathogen to commensal? Longitudinal within-host population dynamics, evolution, and adaptation during a chronic >16-year Burkholderia pseudomallei infection. PLoS Pathog. 2020;16:
e1008298 . DOIPubMedGoogle Scholar - Inglis TJ, Sagripanti JL. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl Environ Microbiol. 2006;72:6865–75. DOIPubMedGoogle Scholar
- Chapple SNJ, Sarovich DS, Holden MTG, Peacock SJ, Buller N, Golledge C, et al. Whole-genome sequencing of a quarter-century melioidosis outbreak in temperate Australia uncovers a region of low-prevalence endemicity. Microb Genom. 2016;2:
e000067 . DOIPubMedGoogle Scholar - Limmathurotsakul D, Holden MT, Coupland P, Price EP, Chantratita N, Wuthiekanun V, et al. Microevolution of Burkholderia pseudomallei during an acute infection. J Clin Microbiol. 2014;52:3418–21. DOIPubMedGoogle Scholar
- McRobb E, Sarovich DS, Price EP, Kaestli M, Mayo M, Keim P, et al. Tracing melioidosis back to the source: using whole-genome sequencing to investigate an outbreak originating from a contaminated domestic water supply. J Clin Microbiol. 2015;53:1144–8. DOIPubMedGoogle Scholar
- Sarovich DS, Chapple SNJ, Price EP, Mayo M, Holden MTG, Peacock SJ, et al. Whole-genome sequencing to investigate a non-clonal melioidosis cluster on a remote Australian island. Microb Genom. 2017;3:
e000117 . DOIPubMedGoogle Scholar - Sagripanti JL, Levy A, Robertson J, Merritt A, Inglis TJ. Inactivation of virulent Burkholderia pseudomallei by sunlight. Photochem Photobiol. 2009;85:978–86. DOIPubMedGoogle Scholar
- Chen YL, Yen YC, Yang CY, Lee MS, Ho CK, Mena KD, et al. The concentrations of ambient Burkholderia pseudomallei during typhoon season in endemic area of melioidosis in Taiwan. PLoS Negl Trop Dis. 2014;8:
e2877 . DOIPubMedGoogle Scholar - Hall CM, Jaramillo S, Jimenez R, Stone NE, Centner H, Busch JD, et al. Burkholderia pseudomallei, the causative agent of melioidosis, is rare but ecologically established and widely dispersed in the environment in Puerto Rico. PLoS Negl Trop Dis. 2019;13:
e0007727 . DOIPubMedGoogle Scholar - Merritt AJ, Inglis TJJ. The role of climate in the epidemiology of melioidosis. Curr Trop Med Rep. 2017;4:185–91. DOIPubMedGoogle Scholar
- Kaestli M, Grist EPM, Ward L, Hill A, Mayo M, Currie BJ. The association of melioidosis with climatic factors in Darwin, Australia: A 23-year time-series analysis. J Infect. 2016;72:687–97. DOIPubMedGoogle Scholar
- Chen PS, Chen YS, Lin HH, Liu PJ, Ni WF, Hsueh PT, et al. Airborne transmission of melioidosis to humans from environmental aerosols contaminated with B. pseudomallei. PLoS Negl Trop Dis. 2015;9:
e0003834 . DOIPubMedGoogle Scholar - Wuthiekanun V, Suputtamongkol Y, Simpson AJ, Kanaphun P, White NJ. Value of throat swab in diagnosis of melioidosis. J Clin Microbiol. 2001;39:3801–2. DOIPubMedGoogle Scholar
- Höger AC, Mayo M, Price EP, Theobald V, Harrington G, Machunter B, et al. The melioidosis agent Burkholderia pseudomallei and related opportunistic pathogens detected in faecal matter of wildlife and livestock in northern Australia. Epidemiol Infect. 2016;144:1924–32. DOIPubMedGoogle Scholar
- Mollaret HH. “The case of the garden of plants” or how melioidosis appeared in France [in French]. Med Mal Infect. 1988;18:643–54. DOIGoogle Scholar
- Hampton V, Kaestli M, Mayo M, Choy JL, Harrington G, Richardson L, et al. Melioidosis in birds and Burkholderia pseudomallei dispersal, Australia. Emerg Infect Dis. 2011;17:1310–2. DOIPubMedGoogle Scholar
- Kaestli M, Mayo M, Harrington G, Ward L, Watt F, Hill JV, et al. Landscape changes influence the occurrence of the melioidosis bacterium Burkholderia pseudomallei in soil in northern Australia. PLoS Negl Trop Dis. 2009;3:
e364 . DOIPubMedGoogle Scholar - Golledge CL, Chin WS, Tribe AE, Condon RJ, Ashdown LR. A case of human melioidosis originating in south-west Western Australia. Med J Aust. 1992;157:332–4. DOIPubMedGoogle Scholar
- Dance DA, King C, Aucken H, Knott CD, West PG, Pitt TL. An outbreak of melioidosis in imported primates in Britain. Vet Rec. 1992;130:525–9. DOIPubMedGoogle Scholar
- Galimand M, Dodin A. Update on melioidosis in the world. Bull Soc Pathol Exot. 1982;75:375–83.
- Sitters H, Minton C, Collins P, Etheridge B, Hassell C, O’Connor F. Extraordinary numbers of oriental pratincoles in NW Australia. Wader Study Group Bulletin. 2004;103:26–31.
Figures
Tables
Cite This ArticleOriginal Publication Date: March 04, 2021
Table of Contents – Volume 27, Number 4—April 2021
| EID Search Options |
|---|
|
|
|
|
|
|


Please use the form below to submit correspondence to the authors or contact them at the following address:
Ella M. Meumann, Menzies School of Health Research, PO Box 41096, Casuarina, NT 0811, Australia
Top