Volume 28, Number 5—May 2022
Research Letter
Cross-Variant Neutralizing Serum Activity after SARS-CoV-2 Breakthrough Infections
Abstract
To determine neutralizing activity against the severe acute respiratory syndrome coronavirus 2 ancestral strain and 4 variants of concern, we tested serum from 30 persons with breakthrough infection after 2-dose vaccination. Cross-variant neutralizing activity was comparable to that after 3-dose vaccination. Shorter intervals between vaccination and breakthrough infection correlated with lower neutralizing titers.
The B.1.1.529 (Omicron) variant of concern of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) carries a high number of nonsynonymous mutations in the spike glycoprotein, relative to that of the ancestral (wild-type) strain (Wu01). Those mutations result in a strong immune evasion phenotype, as demonstrated by severely reduced serum neutralization after vaccination or previous infection with ancestral variants in most persons (1–3), lower vaccine effectiveness, and increased rates of reinfection (N. Andrews et al., unpub. data, https://www.medrxiv.org/content/10.1101/2021.12.14.21267615v1). However, booster vaccinations with 1 dose of mRNA vaccine after priming with an initial 2 doses induce high levels of serum neutralizing activity against Omicron (1,4). Substantial efforts have therefore been made to speed up booster vaccination campaigns in light of the rapid spread of Omicron and the recent surge of infections worldwide. Breakthrough infections after 2-dose mRNA vaccination can result in a natural boost to humoral immunity against SARS-CoV-2 (5; L.J. Abu-Raddad et al., unpub. data, https://www.medrxiv.org/content/10.1101/2022.01.18.22269452v2), and emerging evidence suggests that breakthrough infections with non-Omicron SARS-CoV-2 variants also elicit cross-neutralizing serum activity against Omicron (6).
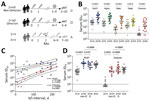
We determined serum neutralizing activity against the spike pseudotypes of SARS-CoV-2 Wu01 strain and 4 variants of concern (Alpha, Beta, Delta, Omicron [BA.1]) in 20 persons with non-Omicron (Alpha, Delta) SARS-CoV-2 infection after 2-dose mRNA vaccination with BNT162b2 (Comirnaty; Pfizer-BioNTech, https://www.comirnaty.com) or heterologous vaccination with ChAdOx1 (Vaxzevria; AstraZeneca, https://www.astrazeneca.com) and BNT162b2 (Appendix). We compared serum neutralization activity for this cohort with that of 2 age-matched cohorts, 1 consisting of 20 persons who received 2 or 3 doses of mRNA vaccine (1) and did not experience breakthrough infection and another cohort of 10 persons who experienced Omicron breakthrough infection after 2-dose vaccination (Figure, panel A; Appendix Table).
We detected significantly higher serum neutralizing activity against all investigated variants in serum from vaccinated persons with subsequent non-Omicron SARS-CoV-2 infection (Figure, panel B) than in serum from persons who received the regular 2 doses of vaccine and experienced no subsequent infection. The geometric mean 50% inhibitory serum dilution (ID50) against Wu01 was 6.3-fold higher after breakthrough infection (640 [95% CI 409–1,003] vs. 4,056 [95% CI 2,174–7,568]). This difference in serum neutralizing activity was particularly pronounced against the Beta (23.5-fold higher ID50, 49 [95% CI 28–85] vs. 1,148 [95% CI 524–2,514]) and Omicron (23.8-fold higher ID50, 9 [95% CI 5–13] vs. 202 [95% CI 79–515]) variants, each of which exhibits substantial immune escape. The boosting effect of non-Omicron breakthrough infections was highly variable (Figure, panel B) because serum neutralizing titers (ID50) showed a strong correlation with the interval between second vaccination and diagnosis of breakthrough infection (Omicron, Spearman ρ = 0.8299, p<0.0001; Wu01, ρ = 0.7048, p = 0.0005) (Figure, panel C; Appendix Figure, panels A–C). Breakthrough infections acquired >3 months after the second vaccination resulted in serum neutralizing capacity against both Wu01 and Omicron, which was comparable to that after 3-dose vaccination. This effect was observed after both non-Omicron and Omicron breakthrough infections (Figure, panel D). Similarly, neutralizing capacity against the Delta variant was increased after Omicron breakthrough infections (Appendix Figure, panel D). Limitations of this study include limited sample size and application of a pseudovirus-based neutralization assay.
In summary, we found that Omicron and non-Omicron SARS-CoV-2 breakthrough infections elicit cross-variant neutralizing antibodies. Our results suggest that short vaccination-to-infection intervals correlate with lower neutralizing titers, which may be relevant for recommendations concerning additional booster vaccination of persons who experience early breakthrough infections after initial immunization.
Dr. Tober-Lau is a physician and researcher at the Department of Infectious Diseases and Respiratory Medicine at Charité–Universitätsmedizin Berlin, Germany. His research interests focus on infectious diseases and global health.
Acknowledgments
We are grateful to all study participants. We also thank Florian Behr, Julia Blum, Annelene Kossow, Göksu Oral, Elham Rezaei, Maike Schlotz, and the members of the EICOV/COVIM Study Group for sample acquisition and processing as well as logistical support: Yvonne Ahlgrimm, Ben Al-Rim, Kerstin Behn, Norma Bethke, Harald Bias, Tobias Bleicker, Dana Briesemeister, Claudia Conrad, Victor Max Corman, Chantip Dang-Heine, Doris Frey, Julie-Anne Gabelich, Janine Gerdes, Ute Gläser, Andreas Hetey, Lisbeth Hasler, Anja Heiduk, Elisa-Theresa Helbig, Alexandra Horn, Claudia Hülso, Stefanie Jentzsch, Luisa Kegel, Paolo Kroneberg, Sebastian Kühn, Irmgard Landgraf, Ngoc Han Le, Michelle Lisy, Lena Johanna Lippert, Constanze Dorothea Lüttke, Pedro de Macedo Gomes, Birgit Maeß, Janine Michel, Friederike Münn, Andreas Nitsche, Anne-Maria Ollech, Christina Pley, Anita Pioch, Annelie Richter, Mira Risham, Carolin Rubisch, Angela Sanchez Rezza, Lisa Ruby, Isabelle Schellenberger, Jenny Schlesinger, Angelika Schliephake, Georg Schwanitz, Tatjana Schwarz, Sevda Senaydin, Alexandra Stege, Sarah Steinbrecher, Paula Stubbemann, Charlotte Thibeault, Denise Treue, and Saskia Zvorc.
This work was supported by grants from COVIM: “NaFoUniMedCovid19” (FKZ: 01KX2021 to L.E.S. and F.K.L.), the Federal Institute for Drugs and Medical Devices (V-2021.3 / 1503_68403 / 2021-2022 to L.E.S. and F.K.U.), the German Center for Infection Research (DZIF) (to F.K.L.), and the Deutsche Forschungsgemeinschaft (DFG) (CRC1310 to F.K.L. and SFB-TR84 to N.S. and L.E.S.). The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
H.G., K.V., and F.K.L. are listed as inventors on pending patent application(s) on SARS-CoV-2-neutralizing antibodies filed by the University of Cologne.
References
- Gruell H, Vanshylla K, Tober-Lau P, Hillus D, Schommers P, Lehmann C, et al. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat Med. 2022; Epub ahead of print. DOIPubMedGoogle Scholar
- Schmidt F, Muecksch F, Weisblum Y, Da Silva J, Bednarski E, Cho A, et al. Plasma neutralization of the SARS-CoV-2 Omicron variant. N Engl J Med. 2022;386:599–601. DOIPubMedGoogle Scholar
- Carreño JM, Alshammary H, Tcheou J, Singh G, Raskin AJ, Kawabata H, et al.; PSP-PARIS Study Group. Activity of convalescent and vaccine serum against SARS-CoV-2 Omicron. Nature. 2022;602:682–8. DOIPubMedGoogle Scholar
- Nemet I, Kliker L, Lustig Y, Zuckerman N, Erster O, Cohen C, et al. Third BNT162b2 vaccination neutralization of SARS-CoV-2 Omicron infection. N Engl J Med. 2022;386:492–4. DOIPubMedGoogle Scholar
- Bates TA, McBride SK, Winders B, Schoen D, Trautmann L, Curlin ME, et al. Antibody response and variant cross-neutralization after SARS-CoV-2 breakthrough infection. JAMA. 2022;327:179–81. DOIPubMedGoogle Scholar
- Rössler A, Riepler L, Bante D, von Laer D, Kimpel J. SARS-CoV-2 Omicron variant neutralization in serum from vaccinated and convalescent persons. N Engl J Med. 2022;386:698–700. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 08, 2022
1These authors contributed equally to this article.
2These authors co-led this study.
Table of Contents – Volume 28, Number 5—May 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Florian Kurth, Charité–Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Department of Infectious Diseases and Respiratory Medicine, Augustenburger Platz 1, D-13353 Berlin, Germany
Top