Volume 28, Number 6—June 2022
Dispatch
Dynamics of SARS-CoV-2 Antibody Response to CoronaVac followed by Booster Dose of BNT162b2 Vaccine
Abstract
We evaluated the longitudinal dynamics of antibody response to the SARS-CoV-2 vaccine CoronaVac and the effect of a booster dose of BNT162b2 vaccine. We found a robust antibody response after the second dose of CoronaVac that wanes over time. The response was recovered by BNT162b2, which boosted anti-spike antibody titers.
Long-term protection against SARS-CoV-2 requires the persistence of vaccine antibodies above protective thresholds, the maintenance of immune memory cells capable of reactivation after subsequent viral exposure, or both (1). A decay of circulating SARS-CoV-2 antibodies over time in persons who received CoronaVac (Sinovac, http://www.sinovac.com) have been reported, suggesting the necessity of a third shot of vaccine (2). In Brazil, the third dose has been administered, preferably, with the BNT162b2 vaccine (Pfizer-BioNTech, https://www.pfizer.com) (3,4). Limited information is available about antibody dynamics after CoronaVac vaccine and the recent supplementing with the BNT162b2 booster. Therefore, we evaluated the longitudinal dynamics of the antibody response to CoronaVac up to 230 days after the second dose in a cohort of healthcare workers (HCWs) and evaluated the effect of a booster dose of BNT162b2 on antibody levels. The study was approved by the Ethics Committee of the Hospital Geral Dr. César Cals (Fortaleza, Brazil; approval no. CAAE 39691420.7.0000.5049). We obtained informed consent from all participants.
We included in this study 99 HCWs of both sexes, >18 years of age, who had received 2 doses of the CoronaVac vaccine, with an interval of 28 days between doses, and then a booster shot of BNT162b2 vaccine 8 months after the second CoronaVac dose. Blood collections and serologic tests were performed at Fundação Oswaldo Cruz (Fiocruz; Ceará, Brazil) and analyzed at 7 different timepoints: before vaccination (P1); 28 days after the first CoronaVac dose (P2); 30 (P3) 90 (P4), 180 (P5), and 230 (P6) days after the second CoronaVac dose; and 15 days after the BNT162b2 dose (P7). We monitored the HCWs for SARS-CoV-2 infection by PCR over time.
We tested all serum samples for IgG against nucleocapsid (N) and spike (S) proteins of SARS-CoV-2 by using chemiluminescent microparticle immunoassays on the ARCHITECT i2000SR equipment (Abbott, https://www.abbott.com). The cutoff value was 50 AU/mL for S antibodies and 1.4 index (S/CO) for N antibodies.
We used GraphPad Prism version 9 (https://www.graphpad.com) for statistical analyses. We describe data as median and interquartile range (IQR) or percentage. In group comparisons, we used χ2 test to analyze the seropositivity data and Kruskal–Wallis test with subsequent Dunn’s test to analyze the IgG values. We considered differences with p<0.05 to be statistically significant.
The cohort was 70.71% women and 29.29% men. Average age was 32.31 years (95% CI 30.3–34.3 years). The age distribution of persons was as follows: 18–30 years, 42 (42.4%); 31–45 years, 51 (51.5%); and >45 years, 6 (6.1%). Median age for each age group was as follows: 18–30 years, 23.0 years (IQR 20–27.3 years); 31–45 years, 36 years (IQR 32–39 years), >45 years, 56.50 years (IQR 52–63.3 years).
Although all HCWs completed the vaccination schedule, some HCWs were unable to give a blood sample in subsequent phases of the study. Therefore, serum samples were obtained from 99 participants in P1 and P2, 95 in P3, 94 in P4, 89 in P5, 84 in P6, and 74 in P7.
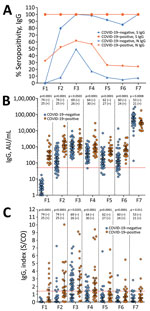
We evaluated the seropositivity and IgG levels for S and N proteins at the different timepoints (Figure 1). At baseline (P1), S IgG were detectable in 25.3% of HCWs, increasing to 84.9% in P2 and reaching 100% in P3. We then observed a decline in seropositivity was observed to 98.9% in P4, 94.4% in P5, and 89.3% in P6. In P7, seropositivity had recovered to 100%. N IgG was detectable in 8.1% of HCWs in P1, 19.2% in P2, and 52.6% in P3, then reduced to 29.8% in P4, 13.5% in P5, 10.7% in P6, and 12.2% in P7 (Figure 1, panel A). The seroconversion rate was 79.7% for S antibodies and 11.9% for N antibodies after the first CoronaVac dose, increasing to 100% for S antibodies and 43.6% N antibodies after the second dose.
After the first CoronaVac dose (P2), S IgG levels were significantly elevated compared with baseline values (p<0.0001) (Figure 1, panel B; Appendix Table 1). Those S IgG levels increased significantly after the second dose (P3) (p<0.0001). However, antibodies levels waned over time. The third BNT162b2 dose again significantly increased S IgG levels (p<0.0001). We observed a similar change in N IgG (Figure 1, panel C; Appendix Table 1). Median values of N IgG were significantly higher after the second CoronaVac dose (p<0.0001) and declined significantly after vaccination (p = 0.0002). In contrast, the third dose with BNT162b2 did not increase N IgG levels.
We evaluated the antibody response to the vaccine in relation to a previous SARS-CoV-2 infection. Twenty-five volunteers were seropositive in P1 and were included in the COVID-19–positive group. Eight volunteers had a positive PCR result during the study (4 in P3, 4 in P4); they were moved to the COVID-19–positive group.
The HCWs who had COVID-19 maintained the anti-S seropositivity at 100% over time. In relation to HCWs who did not have COVID-19, 79.7% of persons became seropositive to S protein in P2. The seropositivity increased to 100% in P3 but decreased in the next timepoints, recovering in P7 (Figure 2, panel A). The differences in seropositivity between the groups were statistically significant in P2 (p = 0.0145). Anti-N seropositivity was 6 (95% CI 2.7–15.1) times higher in the COVID-19–positive group in P2 compared with the COVID-19–negative group. This difference was reduced in P3, increasing in P4 and stabilizing in P5, reaching levels of 5 (95% CI 1.4–18.4) times higher in the COVID-19–positive group.
In the antibody levels analysis, antibody titers for S protein were higher in the COVID-19–positive group than for the COVID-19–negative group at all timepoints except P3 and P7 (Figure 2, panel B; Appendix Table 2). N IgG levels of COVID-19–positive persons were statistically higher than for COVID-19–negative persons at all timepoints (Figure 2, panel C; Appendix Table 2).
We found an antibody response to N and S protein after 2 doses of CoronaVac vaccine. However, the antibodies declined over time. After immunization, the decline of antibodies is expected because not all vaccine-induced plasmablasts commit or are maintained as long-lived memory plasma cells (5). Thus, the success of vaccines depends on the generation and maintenance of immunologic memory (6). Administration of BNT162b2 as the third vaccine dose boosted S IgG but not N IgG. The substantial increase of S IgG after the booster dose suggests that CoronaVac vaccine induced immune memory. The third BNT162b2 dose did not increase the N IgG because mRNA vaccines do not induce a response to the N protein (7,8).
Previously infected participants had a significantly higher antibody level than previously uninfected participants in almost all phases of the study. In addition, we found that those without previous infection showed a faster waning of antibodies over time, a result also reported in previous studies (9). The antibody-making B cells multiply after each exposure, whether attributable to the infection or vaccination; therefore, antibody levels in the previously infected HCWs can reflect the sum of the antibodies produced after infection and vaccine (10).
In summary, a booster dose of BNT162b2 vaccine in HCWs administered 8 months after the second dose with CoronaVac vaccine recalled a specific immune response to SARS-CoV-2. That response had declined substantially 230 days after the second dose of CoronaVac vaccine, resulting in an increase of S IgG after BNT162b2 vaccination and indicating that the 2-dose CoronaVac vaccine schedule generates immune memory.
Dr. Gambim Fonseca is a researcher at and coordinator of the Serology Laboratory of the COVID-19 Diagnosis Support Unit of Fiocruz Ceará, Brazil. Her primary research interests include antibodies and coronavirus disease.
Acknowledgments
We thank the healthcare workers of COVID-19 Diagnosis Support Unit of Fiocruz, Ceará, Brazil, for participating in this study. We also thank the Paulo Goberlânio de Barros Silva for the statistical analysis.
The project is funded by Fiocruz and Ministério da Saúde, Brazil.
References
- Siegrist C-A. Vaccine immunology. 2018 Jan 1 [cited 2022 Mar 22]. https://www.who.int/immunization/documents/Elsevier_Vaccine_immunology.pdf
- Sauré D, O’Ryan M, Torres JP, Zuniga M, Santelices E, Basso LJ. Dynamic IgG seropositivity after rollout of CoronaVac and BNT162b2 COVID-19 vaccines in Chile: a sentinel surveillance study. Lancet Infect Dis. 2022;22:56–63. DOIPubMedGoogle Scholar
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al.; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–15. DOIPubMedGoogle Scholar
- Kanno AI, Barbosa MMF, Moraes L, Leite LCC. SARS-CoV-2 vaccine development and how Brazil is contributing. Genet Mol Biol. 2021;44(Suppl 1):
e20200320 . DOIPubMedGoogle Scholar - Naaber P, Tserel L, Kangro K, Sepp E, Jürjenson V, Adamson A, et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Eur. 2021;10:
100208 . DOIPubMedGoogle Scholar - Palm AE, Henry C. Remembrance of things past: long-term B cell memory after infection and vaccination. Front Immunol. 2019;10:1787. DOIPubMedGoogle Scholar
- Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: A method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;518:9–16. DOIPubMedGoogle Scholar
- Keskin AU, Bolukcu S, Ciragil P, Topkaya AE. SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen. J Med Virol. 2022;94:39–41. DOIPubMedGoogle Scholar
- Vicenti I, Basso M, Gatti F, Scaggiante R, Boccuto A, Zago D, et al. Faster decay of neutralizing antibodies in never infected than previously infected healthcare workers three months after the second BNT162b2 mRNA COVID-19 vaccine dose. Int J Infect Dis. 2021;112:40–4. DOIPubMedGoogle Scholar
- Ali H, Alahmad B, Al-Shammari AA, Alterki A, Hammad M, Cherian P, et al. Previous COVID-19 infection and antibody levels after vaccination. Front Public Health. 2021;9:
778243 . DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: April 14, 2022
Table of Contents – Volume 28, Number 6—June 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcela Helena Gambim Fonseca, Fundação Oswaldo Cruz, São Jose s/n, Eusebio, Ceara, Brazil
Top