Volume 28, Number 6—June 2022
Research Letter
Serum Neutralization of SARS-CoV-2 Omicron BA.1 and BA.2 after BNT162b2 Booster Vaccination
Figure
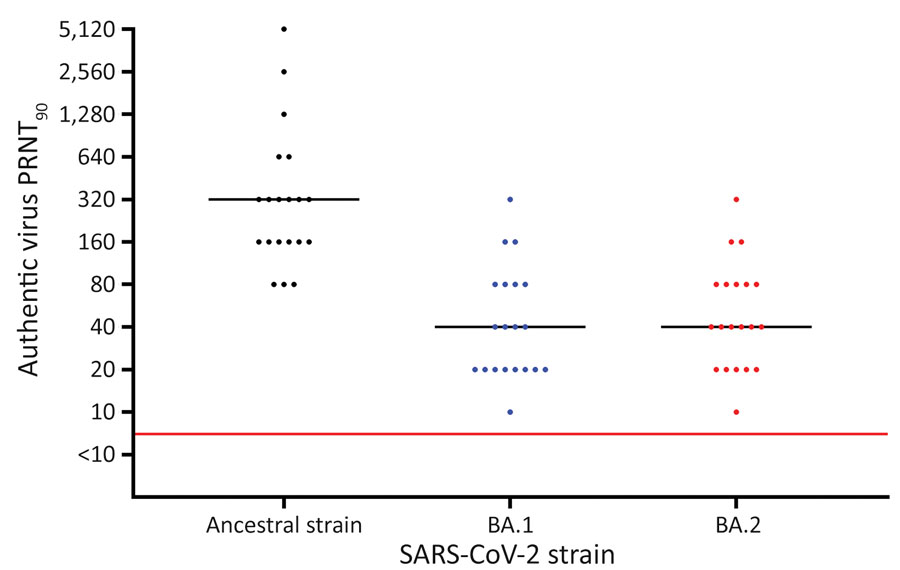
Figure. Results of PRNT90 of serum against SARS-CoV-2 ancestral strain and Omicron sublineages BA.1 and BA.2 after BNT162b2 (Pfizer-BioNTech, https://www.pfizer.com) booster vaccination, Denmark. Serum samples were collected from 20 SARS-CoV-2–naive participants who received 2 BNT162b2 doses and a booster BNT162b2 dose. Viral genome sequences are available in GenBank (accession nos. ON055855 for the ancestral strain, ON055874 for BA.1, and ON055857 for BA.2). Red line indicates neutralization threshold; black lines indicate median neutralization titers for each strain. PRNT90, 90% plaque reduction neutralization test.
Page created: March 30, 2022
Page updated: May 22, 2022
Page reviewed: May 22, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.