Volume 29, Number 11—November 2023
Research
Global Phylogeography and Genomic Epidemiology of Carbapenem-Resistant blaOXA-232–Carrying Klebsiella pneumoniae Sequence Type 15 Lineage
Abstract
Prevalence of carbapenem-resistant Klebsiella pneumoniae (CRKP) has compromised antimicrobial efficacy against severe infections worldwide. To monitor global spread, we conducted a comprehensive genomic epidemiologic study comparing sequences from 21 blaOXA-232–carrying CRKP isolates from China with K. pneumoniae sequence type (ST) 15 strains from 68 countries available in GenBank. Phylogenetic and phylogeographic analyses revealed all blaOXA-232–carrying CRKP isolates belonged to ST15 lineage and exhibited multidrug resistance. Analysis grouped 330 global blaOXA-232–carrying ST15 CRKP strains into 5 clades, indicating clonal transmission with small genetic distances among multiple strains. The lineage originated in the United States, then spread to Europe, Asia, Oceania, and Africa. Most recent common ancestor was traced back to 2000; mutations averaged ≈1.7 per year per genome. Our research helps identify key forces driving global spread of blaOXA-232–carrying CRKP ST15 lineage and emphasizes the importance of ongoing surveillance of epidemic CRKP.
The global spread of carbapenem-resistant Enterobacterales, particularly carbapenem-resistant Klebsiella pneumoniae (CRKP), poses a severe and ongoing public health threat because of high rates of illness and death (1). The production of carbapenemases is the most crucial carbapenem-resistance mechanism in gram-negative bacteria worldwide (2). One of the most prevalent carbapenemase genes, blaOXA-48 (oxacillin-hydrolyzing β-lactamase), is found predominantly on large and transferable plasmids in bacteria of order Enterobacterales. Prevalence of K. pneumoniae strains carrying a blaOXA-48–like gene in China increased during 2018–2022 (3–6). Nosocomial outbreaks, including among pediatric patients, have called attention to the importance of better understanding the transmission potential of CRKP isolates carrying the blaOXA-48–like gene (4–6).
blaOXA-232, a variant among blaOXA-48 genes with limited carbapenem hydrolytic activity, was initially identified in France in 2011 (7). Most blaOXA-232 genes have been discovered on small and nonconjugative ColKP3-type plasmids (8). In China, some strains of sequence type (ST) 15, the predominant lineage of blaOXA-232–carrying K. pneumoniae, mediate low-level carbapenem resistance and many coexist with virulence plasmids (6,9,10). Applying a previously proposed reverse genomic epidemiology strategy enabled us to make genome comparisons of isolates to identify shared sources of infection on the basis of genomic similarity (11). This approach, developed in response to increasing rates of global spread of antimicrobial-resistant bacteria, emphasizes the need to explore transmission of infection from a global rather than a country- or case-specific perspective. Limited data from previous studies on the number and geographic diversity of blaOXA-232–carrying K. pneumoniae isolates has impeded full understanding of the genomic evolution and transmission dynamics of CRKP (5,12).
We aimed to perform a multicenter molecular epidemiologic study of carbapenem-resistant blaOXA-232–carrying K. pneumoniae ST15 isolates in China, focusing on the genomic characterization of the lineage and the genetic context of the blaOXA-232–carrying plasmids. We performed large-scale comprehensive genomic epidemiologic analysis to investigate the transmission histories, common ancestors, evolution rates, and population structures of all publicly available blaOXA-232–carrying K. pneumoniae of ST15 lineage. Our study provides data that will help monitor global spread and the epidemic expansion of K. pneumoniae ST15 lineage and provide insights for developing new infection control strategies. The ethics committee of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, approved this study (2022–0227).
Isolate Collection
We performed a retrospective microbiologic characterization of 21 blaOXA-232–carrying CRKP isolates obtained from a collection of 2,398 nonduplicate K. pneumoniae clinical isolates recovered from 5 hospitals across 3 areas of Zhejiang Province, China, during August 2018–March 2022. Presence of the blaOXA-232 gene was detected by PCR and validated by Sanger sequencing (13). We identified bacteria using VITEK 2 (bioMérieux, https://www.biomerieux.com) and matrix-assisted laser desorption/ionization-time-of-flight (MALDI-TOF) mass spectrometry (Bruker, https://www.bruker.com).
Antimicrobial Susceptibility Testing
We performed antimicrobial susceptibility testing (AST) using a broth microdilution method on antimicrobial agents amikacin, aztreonam, fosfomycin, cefoxitin, cefepime, cefotaxime, cefiderocol, levofloxacin, imipenem, meropenem, polymyxin, tigecycline, and ceftazidime/avibactam. We determined MICs for cefiderocol using iron-depleted cation-adjusted Mueller-Hinton broth in custom-prepared MIC panels (14). We interpreted antimicrobial susceptibility breakpoints according to Clinical and Laboratory Standards Institute 2020 (https://clsi.org) and EUCAST 10.0 (https://www.eucast.org/clinical_breakpoints) guidelines. We used Escherichia coli ATCC 25922 as a quality control strain for AST.
Plasmid Conjugation
We used rifampin-resistant E. coli EC600 as the recipient. We selected transconjugants on Mueller-Hinton agar supplemented with rifampin (300 mg/L) and imipenem (4 mg/L). We counted numbers of transconjugant colonies after overnight incubation at 37°C and confirmed transconjugants using PCR and Sanger sequencing.
Whole-Genome Sequencing
We performed whole-genome sequencing using the short-read Illumina HiSeq X10 (https://www.illumina.com) with 150-bp paired-end protocol. We also sequenced the representative isolate (KP3295) selected from the 21 CRKP isolates on the long-read Oxford Nanopore MinION platform (Nanopore Technologies, https://nanoporetech.com). We assembled the derived short Illumina reads and long MinION reads using Unicycler version 0.4.8 (https://github.com/rrwick/Unicycler) (15).
Genomic Features and Plasmid Characterization
We annotated genomes using the National Center for Biotechnology Information (NCBI) Prokaryotic Genomes Annotation Pipeline (https://github.com/ncbi/pgap). We determined antimicrobial resistance genes using ABRicate version 1.0.1 (https://github.com/tseemann/abricate) against the NCBI AMRFinder database (16) and plasmid replicon types using the PlasmidFinder database (17). We detected virulence determinants using Kleborate version 2.1.0 (18) and the 2022 Virulence Factor Database (19). We performed in silico multilocus sequence typing (MLST) analysis and bacterial source tracking using BacWGSTdb 2.0 (20). We used EasyFigure (21) and BLAST Ring Image Generator (22) to analyze genetic context and homologous regions of blaOXA-232 among the isolates.
Phylogenetic Analysis
On June 3, 2022, we retrieved genome sequences of 43,402 K. pneumoniae strains from 114 countries and related clinical metadata from the NCBI pathogen detection portal (Appendix 1). After conducting in silico MLST analysis and detecting the presence of blaOXA-232 gene in K. pneumoniae, we selected 330 blaOXA-232–carrying K. pneumoniae ST15 strains for further investigation. On the basis of geographic information for each isolate, we created a minimum-spanning tree based on core genome MLST allelic profiles of K. pneumoniae ST15 isolates using chewieSnake (23) and visualized the phylogenetic tree using GrapeTree (24). We considered isolates to be closely related genotypically when separated by a distance of ≤20 alleles. We used Snippy version 4.6.0 (https://github.com/tseemann/snippy) to align sequences and identify single-nucleotide polymorphisms (SNPs). We parsed genome alignment through Gubbins (https://github.com/nickjcroucher/gubbins), which identified and removed recombination regions, and inferred and constructed a maximum-likelihood phylogeny from those SNPs. We calculated pairwise SNP distances between the genomes of each isolate to define clades.
Phylodynamic Analysis
We performed root-to-tip regression analysis using TempEst version 1.5.3 (https://github.com/beast-dev/Tempest) to confirm that the maximum-likelihood tree had sufficient temporal signal. We used a Bayesian Markov Chain Monte Carlo approach implemented in BEAST2 (25) to analyze alignment of putative substitution mutations identified by Gubbins, on which estimates the time-scaled phylogenetic tree was based, as well as time of most recent common ancestor (tMRCA) and mutation rates. We allowed each run 300 million iterations, sampled from every 20,000th iteration, and discarded the first 10% of the samples as burn-in. We estimated the effective sample size using Tracer version 1.7.1 (https://github.com/beast-dev/tracer/releases) to evaluate the operation convergence. We used RhierBAPS version 1.0.1 (26) to analyze the allele frequency parameters of the bacterial population and cluster individual sequences to identify the population structure, then used the resulting clades as input for the SkyGrowth package (https://github.com/mrc-ide/skygrowth) to evaluate effective population sizes. We visualized and annotated phylogenetic trees using the interactive Tree of Life (iTOL) V5 web server (27). We analyzed and visualized transmission links for isolates and nodes with spatial phylogenetic reconstruction of evolutionary dynamics using SpreaD3 (28) under a discrete trait model. We deposited genome sequences of the 21 CRKP isolates in GenBank (BioProject accession nos. PRJNA818898 and PRJNA745926).
Clinical and Microbiologic Characteristics
During August 2018–2022, we recovered blaOXA-232–carrying K. pneumoniae strains from 21 of 2,398 K. pneumoniae patients among 5 hospitals in Zhejiang Province, an incidence rate of 0.87% (Table 1). Most patients with diagnosed K. pneumoniae infections experienced pulmonary infections accompanied by respiratory failure. According to AST data (Table 2), most of the blaOXA-232–positive isolates were resistant to amikacin, aztreonam, fosfomycin, imipenem, meropenem, cefoxitin, cefepime, cefotaxime, and levofloxacin. Those isolates showed low-level resistance to carbapenems but remained susceptible to colistin, tigecycline, ceftazidime/avibactam, and cefiderocol.
Genomic Features and Plasmid Characterization
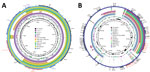
To investigate the genetic characteristics of blaOXA-232–bearing plasmids, we further subjected isolate KP3295 to whole-genome sequencing using the Oxford Nanopore MinION platform. Whole-genome sequencing data revealed that KP3295 consists of a chromosome of 5,329,783 bp and 10 plasmids (177,848 bp, 133,750 bp, 128,536 bp, 9,730 bp, 6,141 bp, 5,640 bp, 4,510 bp, 3,770 bp, and 3,559 bp). The GC (guanine/cytokine) content of KP3295 was 56.82%; the N50 value was 5,329,783 bp. The blaOXA-232 gene was carried by a ColKP3-type plasmid (6,141 bp) designated as pKP3295-5-OXA-232. Among the other 20 blaOXA-232 strains, we identified the blaOXA-232 gene on ColKP3-type plasmids of comparable sizes (5,934–6,141 bp). The plasmid included 9 open reading frames: repA, mobA, mobB, mobC, mobD, ΔISEcp1, blaOXA-232, ΔlysR, and ΔereA (Figure 1). In the conjugation experiment, the blaOXA-232–carrying plasmid could not be transferred to E. coli EC600, which is consistent with the absence of intact conjugal transfer elements in the backbone structure of that plasmid.
Comparative analysis with the genetic surroundings of the 102 blaOXA-232–carrying plasmids in the NCBI nucleotide database revealed that the studied plasmid exhibited 100% coverage and 100% identity to plasmid pOXA-232, originally isolated from E. coli, which marked the initial discovery of blaOXA-232. When carried by the transposable elements ISEcp1 and Tn1000, the compact 6.1 kb blaOXA-232–carrying plasmid could integrate into larger plasmids, such as pOXA-232_30929 (12,351 bp) reported in the Czech Republic and pAI1235M_P5 (9,117 bp) in India. We found the conserved 6.1 kb ColKP3 plasmid structure in association with the transposable element ISEcp1 in IncFIB-ColKP3 (pBio73) and IncFIB-IncHI1B-ColKP3 (pBio45 and pBio3) plasmids from Turkey. Furthermore, with the help of mobile genetic elements, such as ISEcp1 and TnAs1, the ΔISEcp1-blaOXA-232-ΔlysR-ΔereA structure was integrated into plasmids from Bangladesh, specifically IncFIB-IncFII (p_dm730a_NDM5 and 730a-copy-1-OXA-232). Those Inc-type plasmids also carried other β-lactam resistance genes, including blaTEM, bleMBL, blaNDM-1, and blaCTM-X-15 (Figure 1; Figure 2, panel A).
The 21 CRKP isolates carried several virulence determinants involving yersiniabactin (fyuA, ybtE, ybtT, ybtU, irp1, irp2, ybtA, ybtP, ybtQ, ybtX, and ybtS), aerobactin (iutA and iucABCD), and hypermucoidity (rmpA2). All 21 isolates belonged to yersiniabactin sequence type 277 (ybST277) and carried ybt 16. Except for KP232, KP306, and KP77, all isolates belonged to aerobactin sequence type 1 (AbST1) and contained the aerobactin synthesis locus iuc 1. Those virulence determinants were all located on an IncFIB/IncHI1B-type plasmid (177,848 bp), pKP3295-1, in KP3295, which exhibits 99% identity and 91% coverage to classic virulence plasmid pLVPK (Figure 2, panel B).
Occurrence and Divergence of ST15 Lineage
We investigated global phylogenetic relationships of 2,118 K. pneumoniae ST15 strains using core genome MLST analysis with a cutoff of 20 alleles to define clonality (Appendix 2 Table 1). China, the United States, Ireland, and the United Kingdom emerged as the countries with the highest prevalences of ST15. We identified multiple highly homogeneous strains common between countries, notably United States–China (n = 317), China–Nepal (n = 251), United Kingdom–United States (n = 162), and Vietnam–Thailand (n = 124) (Figure 3). Antimicrobial resistance gene testing identified blaKPC-2 (19.07%), blaOXA-232 (15.58%), and blaNDM-1 (10.57%) as the predominant carbapenem-resistance genes carried by global K. pneumoniae ST15 strains.
We identified all 21 blaOXA-232 CRKP isolates collected in this study as ST15. We performed phylogenetic analysis between the 21 blaOXA-232–carrying CRKP isolates and 309 blaOXA-232–carrying K. pneumoniae ST15 isolates obtained from NCBI (Appendix 2 Table 2). The blaOXA-232–carrying K. pneumoniae ST15 isolates originated from 10 different countries: China, United Kingdom, Nepal, Netherlands, Oman, Thailand, United States, France, India, and Australia. Evaluation of clonal relatedness by core-genome SNPs revealed that the blaOXA-232–carrying K. pneumoniae ST15 isolates had an average distance of 29 (range 0–208) SNPs. Phylogenetic analysis using hierarchical Bayesian clustering separated ST15 isolates into 2 clusters, I and II, closely corresponding to China and other countries. Cluster I was further divided into clades 1–4, based on SNP distances ≤20. Among 2 clades from Hangzhou, Zhejiang Province, China, clade 1 had distances of 0–14 (median 4) SNPs and clade 2, 0–20 (median 8) SNPs. Clade 3, primarily from Shanghai, China, had distances of 0–18 (median 10) SNPs. Clade 4, from Hangzhou and Taizhou, Zhejiang Province, showed distances of 0–17 (median 5) SNPs. Strains outside those clades scattered across Shandong Province, Jiangsu Province, Jiaxing in Zhejiang Province, and Shanghai, China, as well as in Nepal. Of note, strains SHK022 and SHK012 from China displayed distances of 13 SNPs, and strains 2703 and 2704 from Nepal, 14 SNPs. Strains from the other 9 countries were predominantly found in cluster II (clade 5), with distances 0–202 (median 100) SNPs.
Subsequently, we analyzed effective population size trajectories for each clade. Clade 1 has steadily ascended since its emergence in 2020. Clade 2 exhibited robust population growth beginning in 2017 and clade 3 in 2016, an increase that projects to clades 2 and 3 dominating global distribution of blaOXA-232–carrying K. pneumoniae ST15 lineage in the future. Clade 4 population sharply declined after emerging, later stabilized, and has increased in recent years. Clade 5 emerged earlier, but its population began to decrease in 2014 (Figure 4).
In-depth phylogenetic analysis revealed details about origins and mutation rates of blaOXA-232–carrying K. pneumoniae ST15 isolates. The correlation coefficient and R2 for the root-to-tip genetic divergence (0.773) compared with time in the TempEst analysis (0.598) indicated a strong linear relationship between accumulated mutations and sampling time, suggesting that enough signal was present to calibrate a strict clock model. The median molecular clock rate was estimated to be 3.40 × 10−3 (95% highest posterior density interval [HPD] 3.10–3.69 × 10−3) substitutions/site/year, which translates to ≈1.7 mutations/genome/year. The tMRCA for blaOXA-232–carrying K. pneumoniae ST15 lineage was estimated from the temporal height, which dates to 2000 (95% HPD 1996–2003). Strains from China appeared within a recent time window (after 2015) but have greatly increased, apparently the result of a rapidly expanding epidemic of clonal transmission.
We also compared carriage of antimicrobial resistance genes and virulence genes by blaOXA-232–carrying K. pneumoniae ST15 strains with strains collected globally. Our findings suggest that the blaOXA-232–carrying K. pneumoniae ST15 strains in the study were multidrug-resistant high-risk clones. The ST15 strains carried several genes that confer resistance to β-lactams (blaOXA-232, blaSHV-106, blaTEM-1, and blaCTX-M-15), aminoglycosides (aac(6′)-Ib, aph(6)-Id, and aph(3″)-Ib), chloramphenicol (catB), quinolones (qnrB1), sulfonamides (sul2), 16S rRNA methylase (rmtF1), trimethoprim (dfrA14), and rifampin (arr2). Most strains also exhibited intrinsic antimicrobial resistance, potentially attributed to genes like fosA6, oqxA6, and oqxB20. Of note, strain KP81 collected in this study harbored 2 carbapenemase genes, blaOXA-232 and blaKPC-2. In addition, this lineage carries various virulence determinants, including yersiniabactin, aerobactin, and hypermucoidity (rmpA2).
Transcontinental Dissemination of Epidemic ST15 Lineage in the 21st Century
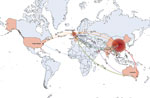
The United States is the likely origin of the ST15 lineage (Figure 5), the offspring of which were introduced into other continents, including Europe, Asia, and Oceania. Transmission from the United States to Europe triggered an epidemic in the United Kingdom. The ST15 lineage was concurrently introduced from the United States to China, where it is the most common strain (93.33%, 308/330 isolates). Epidemic clonal transmissions in China were linked to introductions from the United States (2011–2013), Nepal (2015), and the United Kingdom (2020). Additional global transmission events originated in China and reached the United States, Europe, and Australia. Transmission from China to Australia in 2014 triggered subsequent transmission events from Australia to Europe and Asia. Multiple transmission events occurred from Europe and Australia to Asia in 2015–2016, contributing to complex transmission pathways among countries in Asia. In 2015, strains from Australia reached Oman and subsequently spread to Nepal. In the same year, strains from Nepal spread to Thailand and China. In 2016, strains from Europe were introduced into Thailand and further disseminated to Nepal. After a local outbreak, strains from China spread to India in 2019.
Worldwide spread of CRKP has been accompanied by considerable incidence and death, posing a severe threat to public health (29,30). Multiple nosocomial outbreaks of blaOXA-232–carrying K. pneumoniae have occurred in China and may have contributed to the prevalence of those strains (5,6,9,31). We analysed the genomic features of 21 CRKP ST15 isolates carrying blaOXA-232 collected from various regions in China. We integrated the results with global data to investigate the mode of spread and the possible origins of ST15 blaOXA-232–carrying CRKP. Our findings provide new insights into the genomic characteristics of blaOXA-232–carrying K. pneumoniae and transmission dynamics.
Our analysis of blaOXA-232–carrying CRKP genomes found blaOXA-232 on a 6.1 kb ColKP3 plasmid in isolate KP3295 with high similarity and coverage with previously reported plasmids carrying blaOXA-232 detected worldwide (6,8,13). Our unsuccessful plasmid conjugation experiment demonstrated the presence of blaOXA-232 within a nonconjugative plasmid, confirming findings from several previous studies (7,31). Integration of the blaOXA-232 gene onto IncF-, IncHI1B-, and IncFII-type plasmids through the transposon element ISEcp1 has been documented. IncF- and IncHI1B-type plasmids serve as crucial carriers for multiple antimicrobial resistance genes, such as extended spectrum β-lactamases and blaNDM, that exhibit the ability for interspecies conjugative transfer. Those plasmids are prevalent among members of Enterobacterales and increase risk for wide dissemination (32,33). The integration of ColKP3-type miniplasmid on a larger plasmid suggests that, although not self-transmissible, ColKP3 can be mobilized with the assistance of a self-transmissible plasmid. Of note, plasmids encoding blaOXA-232 are distributed already in South Asia, Europe, and South America. The insertion sequence ISEcp1 was completely lost from the plasmid, which may have inactivated ISEcp1 transposase and ensured the stability of the blaOXA-232 gene on the ColKP3 plasmid (9).
Global dissemination of blaOXA-232 on nonconjugative plasmids, along with escalating outbreaks in China, prompted our investigation into the transmission dynamics and origins of blaOXA-232–carrying K. pneumoniae. A systematic phylogenetic analysis of 2,118 global ST15 strains revealed a high degree of homogeneity among strains originating from different countries, suggesting the dissemination of K. pneumoniae ST15 as a globally prevalent clone. Subsequent genomic epidemiologic analysis revealed distinct regional clustering, primarily within China, characterized by minimal SNP distances, suggesting clonal transmission of blaOXA-232–CRKP ST15 lineage. Clonal transmission may also occur between the strains from China and Nepal based on pairwise distances ≤20 SNPs between these strains. Multiple nosocomial outbreaks of blaOXA-232–CRKP ST15 across different geographic regions also provided epidemiologic evidence to support our investigation (5,6,9). We also collected 1 K. pneumoniae isolate KP81, carrying both blaOXA-232 and blaKPC-2, also reported in the United States in 2020 (34). Emergence of CRKP ST15 carrying both blaOXA-232 and blaKPC-2 in China poses a substantial threat to public health because of its potential for spreading carbapenem resistance internationally through the high-risk clone ST15. blaOXA-232–carrying K. pneumoniae showed low resistance to imipenem and meropenem in our investigation, also described elsewhere (6,9), possibly attributable to disruption of efficient hydrolytic activity because of loss of a salt bridge between residues D159 and R214 (35).
We used Bayesian phylogeny to elucidate the evolutionary history of K. pneumoniae carrying blaOXA-232 within the ST15 lineage. We estimated that this specific lineage appeared in 2000 (95% HPD interval 1989–1998), approximately the same time the ST147 KL10-O3a (2002) and ST258 (1997) lineages were reported (36,37). On the basis of our analysis, we suggest that the ST15 lineage originated in the United States. In addition, we successfully reconstructed early transmission events from the United States to both Europe and Asia. Importated strains from the United States and Nepal to China catalyzed clonal spread of strains already circulating in China. From China, strains of ST15 lineage were disseminated across multiple continents and eventually became globally dispersed. Introduction and inappropriate use of fourth-generation cephalosporin (38) and carbapenem antimicrobial drugs (39,40), approved in clinical settings in the 1990s, may have influenced the global emergence and transmission of ST15 lineage.
Our study was limited by focusing solely on ST15 blaOXA-232–carrying CRKP strains, which we did because those strains have the highest isolation rate in China and have caused multiple nosocomial outbreaks. Future studies are needed to explore global distribution of other lineages. Second, we might have underestimated prevalence of the blaOXA-232 gene because of limited carbapenem hydrolysis activity, possibly resulting in false-negative results in antimicrobial susceptibility testing. Finally, although we comprehensively evaluated all publicly available ST15 blaOXA-232–carrying CRKP strains worldwide, it is possible that some circulating strains went undetected in some regions with dense strain distribution where isolates were derived from a small number of surveillance sites. Therefore, genomic surveillance should be expanded, especially in countries with low isolation rates, to provide a more comprehensive understanding of the emergence, expansion, and spread of CRKP. In conclusion, our research contributes to the comprehension of the global spread of blaOXA-232–carrying CRKP ST15 lineage and emphasizes the need to develop measures for preventing and controlling progression of the CRKP epidemic.
Ms. Wu is a graduate student at Zhejiang University School of Medicine. Her research interests include molecular epidemiology and antimicrobial resistance mechanisms of MDR bacterial pathogens. Dr. Ruan is a principal investigator at Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, China. His primary research interests are microbial genomics and application of bioinformatics to study the genomic epidemiology of multidrug-resistant bacterial infections.
Acknowledgment
This study was supported by the National Natural Science Foundation of China (82102436, 81871696, 82202565), the Zhejiang Provincial Natural Science Foundation of China (LR23H200001, LY21H190001, LQ22H200001), and the Zhejiang Provincial Medical and Health Science and Technology Plan (2020RC066, 2021RC007, 2022RC299, 2023KY227, 2023KY228).
References
- Zhang Y, Wang Q, Yin Y, Chen H, Jin L, Gu B, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62:e01882–17. DOIPubMedGoogle Scholar
- Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9:228–36. DOIPubMedGoogle Scholar
- Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, et al. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART Global Surveillance Program from 2008 to 2014. J Clin Microbiol. 2017;55:1638–49. DOIPubMedGoogle Scholar
- Tian D, Pan F, Wang C, Sun Y, Zhang H. Resistance phenotype and clinical molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae among pediatric patients in Shanghai. Infect Drug Resist. 2018;11:1935–43. DOIPubMedGoogle Scholar
- Li X, Ma W, Qin Q, Liu S, Ye L, Yang J, et al. Nosocomial spread of OXA-232-producing Klebsiella pneumoniae ST15 in a teaching hospital, Shanghai, China. BMC Microbiol. 2019;19:235. DOIPubMedGoogle Scholar
- Han X, Chen Y, Zhou J, Shi Q, Jiang Y, Wu X, et al. Epidemiological characteristics of OXA-232–producing carbapenem-resistant Klebsiella pneumoniae strains isolated during nosocomial clonal spread associated with environmental colonization. Microbiol Spectr. 2022;10:
e0257221 . DOIPubMedGoogle Scholar - Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, et al. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D β-lactamase from Enterobacteriaceae. Int J Antimicrob Agents. 2013;41:325–9. DOIPubMedGoogle Scholar
- Lutgring JD, Zhu W, de Man TJB, Avillan JJ, Anderson KF, Lonsway DR, et al. Phenotypic and genotypic characterization of Enterobacteriaceae producing oxacillinase-48–like carbapenemases, United States. Emerg Infect Dis. 2018;24:700–9. DOIPubMedGoogle Scholar
- Jia H, Zhang Y, Ye J, Xu W, Xu Y, Zeng W, et al. Outbreak of multidrug-resistant OXA-232-producing ST15 Klebsiella pneumoniae in a teaching hospital in Wenzhou, China. Infect Drug Resist. 2021;14:4395–407. DOIPubMedGoogle Scholar
- Shu L, Dong N, Lu J, Zheng Z, Hu J, Zeng W, et al. Emergence of OXA-232 Carbapenemase-producing Klebsiella pneumoniae that carries a pLVPK-like virulence plasmid among elderly patients in China. Antimicrob Agents Chemother. 2019;63:e02246–18. DOIPubMedGoogle Scholar
- Ruan Z, Yu Y, Feng Y. The global dissemination of bacterial infections necessitates the study of reverse genomic epidemiology. Brief Bioinform. 2020;21:741–50. DOIPubMedGoogle Scholar
- Shi Q, Han R, Guo Y, Zheng Y, Yang Y, Yin D, et al. Emergence of ST15 Klebsiella pneumoniae clinical isolates producing plasmids-mediated RmtF and OXA-232 in China. Infect Drug Resist. 2020;13:3125–9. DOIPubMedGoogle Scholar
- Poirel L, Héritier C, Tolün V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48:15–22. DOIPubMedGoogle Scholar
- Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF. Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn Microbiol Infect Dis. 2019;94:321–5. DOIPubMedGoogle Scholar
- Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLOS Comput Biol. 2017;13:
e1005595 . DOIPubMedGoogle Scholar - Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, et al. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother. 2019;63:e00483–19. DOIPubMedGoogle Scholar
- Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother. 2014;58:3895–903. DOIPubMedGoogle Scholar
- Lam MMC, Wick RR, Watts SC, Cerdeira LT, Wyres KL, Holt KE. A genomic surveillance framework and genotyping tool for Klebsiella pneumoniae and its related species complex. Nat Commun. 2021;12:4188. DOIPubMedGoogle Scholar
- Liu B, Zheng D, Zhou S, Chen L, Yang J. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022;50(D1):D912–7. DOIPubMedGoogle Scholar
- Feng Y, Zou S, Chen H, Yu Y, Ruan Z. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49(D1):D644–50. DOIPubMedGoogle Scholar
- Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27:1009–10. DOIPubMedGoogle Scholar
- Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA. BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genomics. 2011;12:402. DOIPubMedGoogle Scholar
- Deneke C, Uelze L, Brendebach H, Tausch SH, Malorny B. Decentralized investigation of bacterial outbreaks based on hashed cgMLST. Front Microbiol. 2021;12:
649517 . DOIPubMedGoogle Scholar - Zhou Z, Alikhan NF, Sergeant MJ, Luhmann N, Vaz C, Francisco AP, et al. GrapeTree: visualization of core genomic relationships among 100,000 bacterial pathogens. Genome Res. 2018;28:1395–404. DOIPubMedGoogle Scholar
- Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A, et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Comput Biol. 2019;15:
e1006650 . DOIPubMedGoogle Scholar - Tonkin-Hill G, Lees JA, Bentley SD, Frost SDW, Corander J. RhierBAPS: An R implementation of the population clustering algorithm hierBAPS. Wellcome Open Res. 2018;3:93. DOIPubMedGoogle Scholar
- Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. DOIPubMedGoogle Scholar
- Bielejec F, Baele G, Vrancken B, Suchard MA, Rambaut A, Lemey P. SpreaD3: interactive visualization of spatiotemporal history and trait evolutionary processes. Mol Biol Evol. 2016;33:2167–9. DOIPubMedGoogle Scholar
- Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55:
105833 . DOIPubMedGoogle Scholar - Xu L, Sun X, Ma X. Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob. 2017;16:18. DOIPubMedGoogle Scholar
- Yin D, Dong D, Li K, Zhang L, Liang J, Yang Y, et al. Clonal dissemination of OXA-232 carbapenemase-producing Klebsiella pneumoniae in neonates. Antimicrob Agents Chemother. 2017;61:e00385–17. DOIPubMedGoogle Scholar
- Yamamoto S, Nakano M, Kitagawa W, Tanaka M, Sone T, Hirai K, et al. Characterization of multi-antibiotic-resistant Escherichia coli Isolated from beef cattle in Japan. Microbes Environ. 2014;29:136–44. DOIPubMedGoogle Scholar
- Villa L, Poirel L, Nordmann P, Carta C, Carattoli A. Complete sequencing of an IncH plasmid carrying the blaNDM-1, blaCTX-M-15 and qnrB1 genes. J Antimicrob Chemother. 2012;67:1645–50. DOIPubMedGoogle Scholar
- Palavecino E, Ramirez K, Greene SR, Kilic A. Co-existence of VIM-2-producing Pseudomonas aeruginosa and KPC-2 and OXA-232-co-producing Klebsiella pneumoniae in the United States. Ann Lab Med. 2020;40:267–9. DOIPubMedGoogle Scholar
- Docquier JD, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, et al. Crystal structure of the OXA-48 beta-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol. 2009;16:540–7. DOIPubMedGoogle Scholar
- Rodrigues C, Desai S, Passet V, Gajjar D, Brisse S. Genomic evolution of the globally disseminated multidrug-resistant Klebsiella pneumoniae clonal group 147. Microb Genom. 2022;8:
000737 . DOIPubMedGoogle Scholar - Gaiarsa S, Comandatore F, Gaibani P, Corbella M, Dalla Valle C, Epis S, et al. Genomic epidemiology of Klebsiella pneumoniae in Italy and novel insights into the origin and global evolution of its resistance to carbapenem antibiotics. Antimicrob Agents Chemother. 2015;59:389–96. DOIPubMedGoogle Scholar
- Long TE, Williams JT. Cephalosporins currently in early clinical trials for the treatment of bacterial infections. Expert Opin Investig Drugs. 2014;23:1375–87. DOIPubMedGoogle Scholar
- Zaffiri L, Gardner J, Toledo-Pereyra LH. History of antibiotics. From salvarsan to cephalosporins. J Invest Surg. 2012;25:67–77. DOIPubMedGoogle Scholar
- Lima LM, Silva BNMD, Barbosa G, Barreiro EJ. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur J Med Chem. 2020;208:
112829 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: October 02, 2023
Table of Contents – Volume 29, Number 11—November 2023
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Zhi Ruan, Jun Zhang or Xinyou Xie, Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, 3 East Qingchun Rd, 310016, Hangzhou, China
Top