Volume 30, Number 2—February 2024
Synopsis
Salmonella Vitkin Outbreak Associated with Bearded Dragons, Canada and United States, 2021–2022
Abstract
We identified 2 cases of Salmonella enterica serovar Vitkin infection linked by whole-genome sequencing in infants in Ontario, Canada, during 2022. Both households of the infants reported having bearded dragons as pets. The outbreak strain was also isolated from an environmental sample collected from a patient’s bearded dragon enclosure. Twelve cases were detected in the United States, and onset dates occurred during March 2021–September 2022 (isolates related to isolates from Canada within 0–9 allele differences by core-genome multilocus sequence typing). Most US patients (66.7%) were <1 year of age, and most (72.7%) had reported bearded dragon exposure. Hospitalization was reported for 5 (38.5%) of 13 patients. Traceback of bearded dragons identified at least 1 potential common supplier in Southeast Asia. Sharing rare serovar information and whole-genome sequencing data between Canada and the United States can assist in timely identification of outbreaks, including those that might not be detected through routine surveillance.
Nontyphoidal Salmonella are consistently among the most commonly reported bacterial pathogens linked to enteric illness in Canada and the United States (1,2). In most cases, human infection is ultimately attributed to consumption of specific foods or to contact with animals (1,3,4). Persons usually show development of illness within 12–36 hours after exposure (range 6 hours–16 days), and most recover without treatment (5,6). However, children <5 years of age, adults >65 years of age, and persons with weakened immune systems are more likely to show development of invasive infections (5–7).
There are >2,600 serovars of Salmonella, more than half of which belong to subspecies I (S. enterica subsp. enterica) and can cause human and animal illness (8–10). Although many Salmonella serovars are predominantly associated with reptiles and amphibians, most of these have low-to-moderate zoonotic potential and are believed to account for <1% of human salmonellosis, mainly affecting persons with weakened immune systems and small children (11). The burden of Salmonella caused by specific serovars varies by country and region, partially because of the distribution of reservoir animal species and because of local food preparation and dietary preferences. Although some serovars, such as Typhimurium and Enteritidis, are globally distributed, others, such as Heidelberg and Newport, are more commonly found in North America (10,12).
In June 2022, Public Health Ontario (PHO) noted 2 cases of a rare Salmonella serovar (Salmonella enterica serovar Vitkin [Salmonella Vitkin]) reported by a local public health unit. The cases were closely related (within 2 alleles based on core-genome multilocus sequence typing [cgMLST]), and onset dates were 4 weeks apart, suggestive of a common exposure.
No cases of Salmonella Vitkin were reported in Ontario or elsewhere in Canada for at least 14 years before these 2 cases (the extent of the data available for review), and the Vitkin serovar had only been seen 23 times in PulseNet USA (https://www.cdc.gov/pulsenet/index.html) before 2021 (13). Similarly, no outbreaks associated with Salmonella Vitkin were documented within the US Centers for Disease Control and Prevention (CDC) National Outbreak Reporting System during 1971–2020 (14).
Given the rarity of this serovar in North America, the Public Health Agency of Canada (PHAC) coordinated communication between PHO and CDC to explore whether CDC had recently identified any cases of this rare serovar in the United States. CDC confirmed that several US isolates of Salmonella Vitkin had been reported to PulseNet USA by multiple US states over the previous year. Analysis of whole-genome sequencing data showed that isolates from the United States and Canada were related within 0–9 cgMLST allele differences. We report the multinational outbreak investigation performed by PHO, PHAC, CDC, and partner agencies to identify the source of this rare outbreak.
Epidemiology
In Ontario, public health units use standardized questionnaires for case interviewing and data collection (15). Using the questionnaire for Salmonella spp., local investigators interviewed a proxy respondent (parent or guardian) for each case and collected data on symptoms and relevant exposures during the 7 days before symptom onset. PulseNet Canada cluster codes for Salmonella spp. are assigned when >2 isolates occur within a 60-day period and are related within 0–10 alleles by whole-genome multilocus sequence typing (wgMLST) (16).
The range of 0–10 wgMLST allele differences might expand or narrow during an investigation on the basis of available laboratory, epidemiologic, and traceback evidence. Wider allele ranges have been previously observed in Salmonella outbreaks linked to zoonotic sources (17,18). Ontario case-patients were defined as patients infected with laboratory-confirmed Salmonella Vitkin who had an isolate matching the PulseNet Canada cluster code (2206VitkinWGS-1ON) by whole-genome sequencing (WGS) and had illness onset during April–September 2022. Data were shared with PHO through the integrated Public Health Information System reporting platform.
In July 2022, PHAC notified CDC of the Ontario case cluster and provided investigators with relevant case demographics, reptile exposure, and laboratory information. CDC queried PulseNet USA, the national molecular subtyping network for foodborne disease surveillance in the United States to confirm relatedness and identify genetically related cases. PulseNet USA cluster codes for Salmonella spp. are typically assigned when >7 clinical isolates occur within a 60-day period and are related within 10 alleles by cgMLST.
US case-patients were defined as patients infected with laboratory-confirmed Salmonella Vitkin that was genetically related within 0–9 allele differences based on cgMLST who had an illness onset of March 2021–September 2022. State and local public health investigators interviewed patients (or their proxies) about possible food or animal exposures before illness onset; those data were shared with CDC through the System for Enteric Disease Response, Investigation, and Coordination platform (19). In September 2022, CDC requested that state health departments collect additional information on bearded dragon husbandry behaviors, purchase locations, and feed.
Laboratory Investigation
We analyzed 4 environmental swab specimens collected from the bearded dragon enclosure in 1 Ontario case household (Figure 1) and a fresh fecal specimen (collected in July 2022) from the bearded dragon (Figure 2) by using routine aerobic culture at the PHO laboratory (20). We also analyzed open samples of dried, pelleted reptile feed and a reptile calcium supplement by routine culture at the PHO laboratory (20). PHO runs WGS for all S. enterica by using the standardized PulseNet Canada protocol (21). WGS data are analyzed locally and then uploaded to a centralized BioNumerics version 7.6 (bioMérieux, https://www.biomerieux.com) database, where it is compared with data from other jurisdictions in Canada to identify multijurisdictional clusters by using wgMLST. PulseNet Canada uses wgMLST as a primary method for identifying genetically related isolates but also has the ability to use cgMLST for comparison with other jurisdictions that use this method. Canada and the United States routinely share genomic data under a bilateral information sharing agreement for comparison between the 2 countries because Canada does not yet upload WGS data to a public repository, such as the National Center for Biotechnology Information (NCBI; https://www.ncbi.nlm.nih.gov), in real time.
In this investigation, sequence data from a representative Salmonella Vitkin isolate was requested from PulseNet USA and added to the Canada database, where it was determined to be related to the Ontario cases within 1–3 cgMLST allele differences. A representative sequence from Canada was shared with PulseNet USA for comparison by cgMLST to identify related isolates in the United States. For Canada isolates, WGS data were deposited retrospectively in NCBI in the PulseNet Canada Salmonella BioProject (PRJNA543337). US sequences were uploaded to NCBI under the PulseNet Salmonella BioProject (PRJNA230403).
We reviewed veterinary isolate data, representing specimens collected from sick or deceased reptiles and tested by the Animal Health Laboratory (AHL) in Ontario during 2013–2022 to determine whether Salmonella Vitkin had been detected in reptiles in Ontario by this surveillance program. Reptile species tested at the AHL reflect common pet species as well as samples from private zoologic institutions. No US patients permitted investigators to screen their bearded dragons for Salmonella spp.
Traceback of Reptiles
We conducted a traceback investigation for each of the patient’s bearded dragons to identify a potential common supplier or breeder. Where applicable, local pet stores, and intermediary suppliers were interviewed to obtain information on the source(s) of their reptiles and whether they were acquired by local breeders or imported.
Ethics
This study did not require research ethics committee approval because activities described herein were conducted in fulfillment of the legislated mandate of PHO “to provide scientific and technical advice and support to the health care system and the Government of Ontario in order to protect and promote the health of Ontarians” and are therefore considered public health practice, not research (22). Similarly, this activity was conducted consistent with applicable federal law and CDC policy (see e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.).
Epidemiology
Both Ontario case-patients were <1 year of age and had isolates related within 3 alleles. Onset dates were reported to be ≈4 weeks apart, in April and May 2022. No hospitalization or death was reported for either of the Ontario patients.
We did not identify any common food exposures (including infant formula) among the Ontario patients. However, the proxy respondent for each patient reported >1 bearded dragons in the household (Table). We noted no commonalities between households with respect to bearded dragon diet. One household reported feeding their reptile(s) feeder mice.
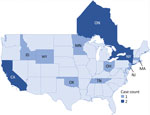
Twelve cases of Salmonella Vitkin were identified in the United States, were considered genetically related to the outbreak strain, and had illness onset dates ranging from March 2021 to September 2022 (Figure 3). The US cases were identified across 10 states (Figure 4). Of 11 patients with additional information available, 5 (45%) were hospitalized and no deaths were reported. The median age of US case-patients was the same as that of the Ontario case-patients, including 8 (67%) children <1 year of age (Table).
Among the 11 US patients who had available exposure information, 8 (73%) reported either having direct contact with bearded dragons or having one in their household (Table). No common food exposures were identified among patients.
Laboratory Investigation
One environmental swab specimen, collected from the entrance to the bearded dragon’s rock cave (Figure 2), was positive for Salmonella Vitkin. Two isolates from this swab specimen were related to the outbreak strain by WGS (Figure 3). Salmonella spp. were not detected from the other 3 environmental swab specimens, the fresh fecal specimen collected from the same reptile enclosure, the open sample of reptile feed, or the reptile calcium supplement. Canada human isolates and 2 environmental isolates were genetically related within 0–3 cgMLST allele differences; they also were genetically related within 0–9 cgMLST allele differences to 12 US clinical cases. The 4 Canada Salmonella Vitkin isolates linked to this investigation were the only Vitkin isolates in the Canada database. Therefore, no additional strains were analyzed outside the US isolates.
Traceback of Reptiles
Ontario patients reported purchasing bearded dragons from 2 different pet store locations in Ontario, and US patients reported purchases from 4 different US pet store locations and an online source. Both Ontario pet stores were supplied by a single common intermediary supplier, which imported bearded dragons from various suppliers, including an international supplier located in Southeast Asia. This intermediary supplier reported that they did not ship bearded dragons from or to the United States and that they had ceased importing reptiles from the international supplier in late 2021. The US pet stores were supplied by 1 of 3 common intermediary suppliers, 1 of which purchased bearded dragons from the same international supplier. No single breeder was identified as the source for all bearded dragons implicated in this investigation.
Reptile Isolate Data
A review of reptile veterinary isolate data compiled by the AHL found that, although most of the specimens tested from 2013–2022 were from bearded dragons (by reptile species, 21.3%, n = 13), Salmonella Vitkin was not isolated from any of these, or from any other reptile species. The serovars most commonly isolated from bearded dragons were Salmonella Amsterdam (23.1%, n = 3) and Salmonella Kisarawe (23.1%, n = 3).
Public Health Response
Local public health investigators visited each pet store identified by the Ontario case-patients as the location of bearded dragon purchase and provided pet store operators with fact sheets summarizing information on Salmonella infection and prevention associated with reptiles for distribution to future customers. Information provided by fact sheets included recommendations for pet owners to wash their hands after handling reptiles, to clean and disinfect any surfaces that come into contact with their reptile, and to supervise children during interactions with reptiles, while noting that these animals are not recommended as pets for households with >1 persons at increased risk for severe illness (23). Canada and US public health partners shared their outbreak findings with public health officials located in the country of the common Southeast Asia bearded dragon supplier, as well as educational resources on preventing Salmonella transmission from bearded dragons.
CDC first communicated to the public regarding this outbreak on October 18, 2022. The website post highlighted investigation details and information for actions to take to minimize the risk for reptile-associated Salmonella infections (24). Similar general preventative information in the form of a public-facing factsheet is available on the PHAC webpage (23). The investigations in Canada and the United States were closed in December 2022.
Salmonella Vitkin infections linked to this outbreak disproportionately affected infants and often resulted in severe illness, as shown by 78% of reported case-patients being <1 year of age and almost 40% of patients reporting hospitalization because of illness. Contact with bearded dragons was the source of this outbreak. Reporting of indirect or direct contact with bearded dragons by most case-patients and isolation of the same serovar from a reptile enclosure supported bearded dragons as the most likely source of exposure. Many infants likely were indirectly exposed to infection by the contaminated clothing or hands of caregivers or by contact with contaminated environmental surfaces within the home.
Reptiles, including bearded dragons, lizards and snakes, are among several animal species that are becoming more common in Canada and elsewhere as household pets (25–27). Reptiles can carry Salmonella spp. in their gastrointestinal tract without displaying signs of illness and can intermittently shed the bacteria in their feces (28). Fecal shedding can be prompted or exacerbated by stressors, such as handling, transportation, or illness (28). Persons might become ill with Salmonella infections if they have direct or indirect contact with reptiles or their environment, particularly if they do not wash their hands after handling or caring for their reptiles or if they do not clean and disinfect contaminated surfaces (26,29).
A study found that, although most cases of human salmonellosis in Ontario during 2010–2012 were attributed to foodborne transmission, 35.5% (n = 107) of reported cases were attributed to contact with reptiles or amphibians (3). This finding is notable because, according to the most recent Canadian Foodbook Report, produced by the Public Health Agency of Canada in 2015 after a population-based telephone survey of the Canadian population, only 2.1% of Ontario respondents reported having any contact with reptiles in the previous 7 days, indicating the relatively small proportion of the population who might keep these reptiles as pets (30). In comparison, 31.0% of Ontario respondents reported having contact with a cat and 42.2% reported having contact with a dog during the same time period (30). Similarly, a recent national pet owner survey found that ≈90.5 million US homes reported owning >1 pet. Among households that owned a pet, ≈6% (5.7 million) owned a reptile, compared with 76% (69.0 million) that owned a dog and 50% (45.3 million) that owned a cat (31).
Captive bearded dragons have higher rates of Salmonella carriage compared with those in the wild, and stress can increase the frequency of Salmonella shedding (32). Awareness and consideration of steps that can be taken by owners and breeders of bearded dragons to reduce stressors (such as avoiding overcrowding during transport, and provision of adequate space and enrichment items within enclosures) could theoretically reduce Salmonella shedding and subsequent human exposure (33). Bearded dragon owners should be encouraged to restrict roaming of their bearded dragons outside of their enclosure to surfaces and items that are able to be cleaned and disinfected afterwards.
If reptile owners are not aware that their reptiles can carry and shed Salmonella spp., they might fail to take appropriate preventive measures, increasing the risk for illness among household members, including those who do not have direct contact with reptiles. Because some Salmonella serovars can survive on surfaces for several days to months, surfaces might serve as a source of indirect exposure if they are not cleaned and disinfected after coming into contact with bearded dragons or other reptiles (34,35). Previous studies have noted that Salmonella carriage is common among captive reptiles and that a high-density population of animals (e.g., in breeding facilities, during transport or at point of sale) can promote the transfer of Salmonella spp. between reptiles, particularly if the reptiles are fed with rodents (26,36).
There have been several reported outbreaks of Salmonella infections linked to contact with reptiles in Canada and the United States, including outbreaks specifically linked to bearded dragons. Those outbreaks have involved several different serovars, including Uganda (2022), Muenster (2020), Apapa (2018), and Cotham (2014) (27,37–40). Although those outbreaks affected persons of all age groups, children were overrepresented.
To date, there has been minimal published literature on Salmonella Vitkin infections in humans; we identified only 1 article summarizing a case of meningitis caused by Salmonella Vitkin infection in an infant (1 month of age) after exposure to a pet turtle (29). Children, particularly those <5 years of age, might be at increased risk for exposure to Salmonella infections and other enteric infections because of poor hand hygiene, developing immune systems, and a tendency to mouth objects (41). Children might also be more susceptible to less virulent strains of the bacteria, which might explain the relatively young median age of those involved in reported outbreaks involving reptiles (25). Because transmission can occur by direct and indirect contact with reptiles, parents might not recognize indirect reptile contact as a risk. As such, they might fail to take appropriate preventive measures, including keeping children away from the reptiles and any potentially contaminated surfaces. Furthermore, parents and caregivers might not think to change potentially contaminated clothing or wash their hands between handling reptiles and interacting with children (11). Infants could be at increased risk for indirect exposure by the clothing of adult household members because they are more likely to be held or carried than independently mobile toddlers and older children.
Although a fresh fecal specimen from 1 bearded dragon was collected during the Salmonella Vitkin investigation and found to be negative for Salmonella spp., this specimen was collected 3 months after the onset of illness in the child within the same household, and the bearded dragon might have no longer (or only intermittently) been shedding Salmonella spp. in its stool at the time. A previous study of household Salmonella transmission associated with pet reptiles in Germany found that in 15 (78.9%) of 19 households with a child with confirmed Salmonella infection, an identical serovar was confirmed in both the infected case and >1 reptile in the household (42). The authors further noted that, although reptiles might be simultaneously colonized with multiple Salmonella serovars, shedding may be intermittent, and a negative cloacal or fecal specimen does not mean that the reptile is free from Salmonella spp. (42). Instead, persons should assume that all reptiles could be carrying Salmonella spp. (42).
Although the intermediary reptile supplier in Canada in this investigation did not report US importation or exportation of reptiles, it is unknown whether cross-border importation or export of reptiles is common practice or the extent to which importation of reptiles to North America might occur. Ontario has no record-keeping requirements for persons who breed, import, export or sell reptiles. Bearded dragons within the pet trade are entirely maintained by captive breeding operations that might operate at a small scale (i.e., a person with 2 bearded dragons) or at a commercial scale (33). The stress and close confinement during transport is associated with an increased risk for Salmonella shedding and transmission (11). Although cases of Salmonella Vitkin infection linked to this outbreak were reported in the United States until November 2022, no additional cases were reported in Ontario (or elsewhere in Canada) after the intermediary supplier in Canada ceased importing bearded dragons from the common international breeder that was identified in this investigation.
Because reptile supply chains in Canada and the United States might be integrated for some species, communication between international public health investigators can assist in identification of multijurisdictional outbreaks associated with reptiles and can help to identify a potentially causative exposure, particularly in situations with rare serovars. Furthermore, environmental sampling can provide microbiological evidence to confirm source identification.
Providing reptile owners with information on the risks of Salmonella infection associated with reptiles at the point of purchase/acquisition could support informed decisions about pet choices and necessary precautions. Information could include steps that can be taken to minimize the risk for infection transmission from reptiles to humans, including details on persons who might be at increased risk for illness if exposed and who should ideally avoid reptile contact (43). In particular, potential reptile owners who have children <5 years of age should be aware that reptile ownership or contact is discouraged for this age group because, although handwashing and environmental disinfection can reduce the risk for Salmonella transmission from reptiles to humans, the increased susceptibility of children to infection and the risk for severe illness if infected make these high-risk pets. Further education for persons and businesses involved in reptile breeding, distribution, and sale could also focus on the need for preventive veterinary care, biosecurity, and environmental cleaning practices (43).
Record-keeping requirements for persons involved in the breeding, distribution, and sale of reptiles would assist in traceback investigations and support investigators in identifying the source of infection during outbreak investigations (43). Timely collaboration and information sharing can assist in identifying the potential source of a multijurisdictional outbreak, enabling rapid rollout of public health interventions and dissemination of information to the public to prevent illnesses.
Dr. Paphitis is the Enteric Zoonotic Specialist at Public Health Ontario, Toronto, Ontario, Canada. Her primary research interests are One Health and the epidemiology of enteric and zoonotic diseases, including salmonellosis.
Acknowledgment
We thank all persons involved in case investigation, case follow-up, and reptile traceback, including state/local public health partners in California, Idaho, Massachusetts, Minnesota, New Jersey, New York, Ohio, Oklahoma, Tennessee, and Wyoming, and particularly the Ontario public health unit investigators who conducted a comprehensive investigation, including environmental sampling; the Public Health Ontario laboratory for their work isolating, identifying, and sequencing Salmonella during this outbreak; Tim Pasma for providing the reptile isolate data used for reference in this outbreak; and Lauren Gollarza and Kaylea Nemechek for providing communication and epidemiologic technical input on enteric zoonoses during this investigation.
References
- Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17:7–15. DOIPubMedGoogle Scholar
- Thomas MK, Murray R, Flockhart L, Pintar K, Fazil A, Nesbitt A, et al. Estimates of foodborne illness-related hospitalizations and deaths in Canada for 30 specified pathogens and unspecified agents. Foodborne Pathog Dis. 2015;12:820–7. DOIPubMedGoogle Scholar
- Whitfield Y, Johnson K, Hobbs L, Middleton D, Dhar B, Vrbova L. Descriptive study of enteric zoonoses in Ontario, Canada, from 2010 - 2012. BMC Public Health. 2017;17:217. DOIPubMedGoogle Scholar
- Beshearse E, Bruce BB, Nane GF, Cooke RM, Aspinall W, Hald T, et al. Attribution of illnesses transmitted by food and water to comprehensive transmission pathways using structured expert judgment, United States. Emerg Infect Dis. 2021;27:182–95. DOIPubMedGoogle Scholar
- Heymann DL. Control of communicable diseases manual. 20th ed. Washington: American Public Health Association; 2015.
- Shane AL, Mody RK, Crump JA, Tarr PI, Steiner TS, Kotloff K, et al. 2017 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and management of infectious diarrhea. Clin Infect Dis. 2017;65:e45–80. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Salmonella: information for healthcare professionals and laboratories [cited 2023 Jan 12]. https://www.cdc.gov/salmonella/general/technical.html
- Jajere SM. A review of Salmonella enterica with particular focus on the pathogenicity and virulence factors, host specificity and antimicrobial resistance including multidrug resistance. Vet World. 2019;12:504–21. DOIPubMedGoogle Scholar
- Guibourdenche M, Roggentin P, Mikoleit M, Fields PI, Bockemühl J, Grimont PA, et al. Supplement 2003-2007 (No. 47) to the White-Kauffmann-Le Minor scheme. Res Microbiol. 2010;161:26–9. DOIPubMedGoogle Scholar
- Ferrari RG, Rosario DKA, Cunha-Neto A, Mano SB, Figueiredo EES, Conte-Junior CA. Worldwide epidemiology of Salmonella serovars in animal-based foods: a meta-analysis. Appl Environ Microbiol. 2019;85:e00591–19. DOIPubMedGoogle Scholar
- Hoelzer K, Moreno Switt AI, Wiedmann M. Animal contact as a source of human non-typhoidal salmonellosis. Vet Res (Faisalabad). 2011;42:34. DOIPubMedGoogle Scholar
- Hendriksen RS, Vieira AR, Karlsmose S, Lo Fo Wong DM, Jensen AB, Wegener HC, et al. Global monitoring of Salmonella serovar distribution from the World Health Organization global foodborne infections network country data bank: results of quality assured laboratories from 2001 to 2007. Foodborne Pathog Dis. 2011;8:887–900. DOIPubMedGoogle Scholar
- Tolar B, Joseph LA, Schroeder MN, Stroika S, Ribot EM, Hise KB, et al. An overview of PulseNet USA databases. Foodborne Pathog Dis. 2019;16:457–62. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. National Outbreak Reporting System (NORS) [cited 2022 Dec 18]. https://wwwn.cdc.gov/norsdashboard
- Ontario Agency for Health Protection and Promotion (Public Health Ontario). Ontario investigation tools (OIT). Toronto: King’s Printer for Ontario; 2019 Dec [cited 2022 Dec 6]. https://www.publichealthontario.ca/en/diseases-and-conditions/infectious-diseases/ccm/oit
- Public Health Agency of Canada. Outbreak toolkit: cluster code; 2020 [cited 2022 Dec 6]. https://outbreaktools.ca/case-study-overview/module-1-cluster-detection-and-initial-investigation-2/cluster-code
- Fagan-Garcia K, Denich L, Tataryn J, Janicki R, Van Osch O, Kearney A, et al. A multi-provincial Salmonella Typhimurium outbreak in Canada associated with exposure to pet hedgehogs, 2017-2020. Can Commun Dis Rep. 2022;48:282–90. DOIPubMedGoogle Scholar
- Gerner-Smidt P, Besser J, Concepción-Acevedo J, Folster JP, Huffman J, Joseph LA, et al. Whole genome sequencing: bridging One-Health surveillance of foodborne diseases. [Erratum in: Front Public Health. 2019;7:365.]. Front Public Health. 2019;7:172. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. SEDRIC: System for Enteric Disease Response, Investigation and Coordination [cited 2023 Jan 17]. https://www.cdc.gov/foodsafety/outbreaks/tools/sedric.html
- Reed A. Isolation and identification of Salmonella from foods and environmental samples. MFHPB-20. Health Products and Food Branch, Food Directorate, Health Canada; 2009 [cited 2023 Jan 13]. https://www.canada.ca/en/health-canada/services/food-nutrition/research-programs-analytical-methods/analytical-methods/compendium-methods/methods-microbiological-analysis-foods-compendium-analytical-methods.html
- Rumore J, Tschetter L, Kearney A, Kandar R, McCormick R, Walker M, et al. Evaluation of whole-genome sequencing for outbreak detection of Verotoxigenic Escherichia coli O157:H7 from the Canadian perspective. BMC Genomics. 2018;19:870. DOIPubMedGoogle Scholar
- Ontario Agency for Health Protection and Promotion Act. SO 2007, c 10 s 6. Sect. c 10, s 6 Jun 4, 2007 [cited 2023 Dec 19]. https://www.canlii.org/en/on/laws/stat/so-2007-c-10-sch-k/latest/so-2007-c-10-sch-k.html
- Public Health Agency of Canada. Salmonella and reptiles. 2022 [cited 2022 Dec 13]. https://www.canada.ca/en/public-health/services/food-safety/fact-sheet/salmonella-reptiles.html
- Centers for Disease Control and Prevention. Salmonella homepage. 2022. Salmonella outbreak linked to pet bearded dragons (S. Vitkin) [cited 2022 Dec 5]. https://www.cdc.gov/salmonella/beardeddragon-10-22/details.html
- Harker KS, Lane C, De Pinna E, Adak GK. An outbreak of Salmonella Typhimurium DT191a associated with reptile feeder mice. Epidemiol Infect. 2011;139:1254–61. DOIPubMedGoogle Scholar
- Zając M, Skarżyńska M, Lalak A, Kwit R, Śmiałowska-Węglińska A, Pasim P, et al. Salmonella in captive reptiles and their environment—can we tame the dragon? Microorganisms. 2021;9:1012. DOIPubMedGoogle Scholar
- Valdez JW. Using Google trends to determine current, past, and future trends in the reptile pet trade. Animals (Basel). 2021;11:676. DOIPubMedGoogle Scholar
- Bjelland AM, Sandvik LM, Skarstein MM, Svendal L, Debenham JJ. Prevalence of Salmonella serovars isolated from reptiles in Norwegian zoos. Acta Vet Scand. 2020;62:3. DOIPubMedGoogle Scholar
- Ricard C, Mellentin J, Ben Abdallah Chabchoub R, Kingbede P, Heuclin T, Ramdame A, et al. [Salmonella meningitis in an infant due to a pet turtle] [in French]. Arch Pediatr. 2015;22:605–7. DOIPubMedGoogle Scholar
- Public Health Agency of Canada. Foodbook report. Ottawa (ON, Canada): Government of Canada; 2015 Nov [cited 2022 Dec 6]. https://www.canada.ca/en/public-health/services/publications/food-nutrition/foodbook-report.html
- American Pet Products Association. Pet industry market size, trends and ownership statistics. 2022 [cited 2022 Dec 16]. https://www.americanpetproducts.org/press_industrytrends.asp
- Whiley H, Gardner MG, Ross K. A review of Salmonella and squamates (lizards, snakes and amphibians): implications for public health. Pathogens. 2017;6:38. DOIPubMedGoogle Scholar
- Johnson R, Adwick S. Central bearded dragons (Pogona vitticeps). In: Yeates J, editor. Companion animal care and welfare. Chichester (UK): John Wiley & Sons, Ltd; 2018. p. 395–411.
- Bauwens L, Vercammen F, Bertrand S, Collard JM, De Ceuster S. Isolation of Salmonella from environmental samples collected in the reptile department of Antwerp Zoo using different selective methods. J Appl Microbiol. 2006;101:284–9. DOIPubMedGoogle Scholar
- Pedersen K, Lassen-Nielsen AM, Nordentoft S, Hammer AS. Serovars of Salmonella from captive reptiles. Zoonoses Public Health. 2009;56:238–42. DOIPubMedGoogle Scholar
- Lee KM, McReynolds JL, Fuller CC, Jones B, Herrman TJ, Byrd JA, et al. Investigation and characterization of the frozen feeder rodent industry in Texas following a multi-state Salmonella Typhimurium outbreak associated with frozen vacuum-packed rodents. Zoonoses Public Health. 2008;55:488–96. DOIPubMedGoogle Scholar
- Government of Canada. Public health notice: outbreak of Salmonella infections linked to snakes and rodents. 2021 [cited 2022 Dec 5]. https://www.canada.ca/en/public-health/services/public-health-notices/2019/outbreak-salmonella-infections-snakes-rodents.html
- Kiebler CA, Bottichio L, Simmons L, Basler C, Klos R, Gurfield N, et al. Outbreak of human infections with uncommon Salmonella serotypes linked to pet bearded dragons, 2012-2014. Zoonoses Public Health. 2020;67:425–34. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Reports of selected Salmonella outbreak investigations. 2022 [cited 2023 Jan 26]. https://www.cdc.gov/salmonella/outbreaks.html
- Waltenburg MA, Perez A, Salah Z, Karp BE, Whichard J, Tolar B, et al. Multistate reptile- and amphibian-associated salmonellosis outbreaks in humans, United States, 2009-2018. Zoonoses Public Health. 2022;69:925–37. DOIPubMedGoogle Scholar
- Sockett PN, Rodgers FG. Enteric and foodborne disease in children: A review of the influence of food- and environment-related risk factors. Paediatr Child Health. 2001;6:203–9. DOIPubMedGoogle Scholar
- Pees M, Rabsch W, Plenz B, Fruth A, Prager R, Simon S, et al. Evidence for the transmission of Salmonella from reptiles to children in Germany, July 2010 to October 2011. Euro Surveill. 2013;18:20634. DOIPubMedGoogle Scholar
- Varela K, Brown JA, Lipton B, Dunn J, Stanek D, Behravesh CB, et al.; NASPHV Committee Consultants, et al. A review of zoonotic disease threats to pet owners: a compendium of measures to prevent zoonotic diseases associated with non-traditional pets such as rodents and other small mammals, reptiles, amphibians, backyard poultry, and other selected animals. Vector Borne Zoonotic Dis. 2022;22:303–60. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 30, Number 2—February 2024
| EID Search Options |
|---|
|
|
|
|
|
|



Please use the form below to submit correspondence to the authors or contact them at the following address:
Katherine Paphitis, Public Health Ontario, 661 University Ave, Ste 1701, Toronto, ON M5G 1M1, Canada
Top