Volume 30, Number 3—March 2024
Research
Estimates of Incidence and Predictors of Fatiguing Illness after SARS-CoV-2 Infection
Abstract
This study aimed to estimate the incidence rates of post–COVID-19 fatigue and chronic fatigue and to quantify the additional incident fatigue caused by COVID-19. We analyzed electronic health records data of 4,589 patients with confirmed COVID-19 during February 2020–February 2021 who were followed for a median of 11.4 (interquartile range 7.8–15.5) months and compared them to data from 9,022 propensity score–matched non–COVID-19 controls. Among COVID-19 patients (15% hospitalized for acute COVID-19), the incidence rate of fatigue was 10.2/100 person-years and the rate of chronic fatigue was 1.8/100 person-years. Compared with non–COVID-19 controls, the hazard ratios were 1.68 (95% CI 1.48–1.92) for fatigue and 4.32 (95% CI 2.90–6.43) for chronic fatigue. The observed association between COVID-19 and the significant increase in the incidence of fatigue and chronic fatigue reinforces the need for public health actions to prevent SARS-CoV-2 infections.
According to the Household Pulse Survey conducted by the US Centers for Disease Control and Prevention in January 2023, up to 15% of all US adults had experienced >1 symptoms of post–COVID-19 conditions (PCC), also known as long COVID or postacute sequelae of SARS-CoV-2 infection (PASC) (1). Among persons with PCC, fatigue is frequently reported in both hospitalized and nonhospitalized patients (2,3). A recent prospective cohort study reported 85% of patients who met its PASC definition had fatigue (4). A substantial percentage of patients with fatigue remain ill for many months with an illness similar to myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) (5), an unexplained syndrome sometimes seen after infections that is characterized by functional limitations that impair patients’ ability to maintain daily activities and is associated with profound fatigue (6).
The burden, distribution, and trend of PCC can theoretically be measured by using prevalence and incidence. The prevalence of PCC is a useful measure of overall disease burden at a specific time but is dependent on recovery, deaths, and incidence. The incidence of PCC measures the rate of new cases over a certain period and can be valuable for informing public health actions to reduce new illnesses. Numerous studies have estimated PCC prevalence, but very few have attempted to estimate PCC incidence because the incidence estimate requires information on timing of incident event and a well-defined population at risk that does not include prevalent cases (7). Both requirements are challenging in the context of PCC because they consist of a range of conditions and symptoms, most of which are not specific to PCC. To date, no diagnostic biomarkers are available, and recognition of PCC requires integrating medical history and clinical findings. Recent studies also emphasize the importance of an equivalent, concurrent, non–COVID-19 comparison group so that the effects of COVID-19 will not be overestimated (8). Given the central role of fatigue in PCC and the lack of data on incidence of fatigue among patients who have had COVID-19, we conducted a study of incident fatigue diagnoses among patients with and without COVID-19. Our objectives were to estimate the incidence rates of fatigue and chronic fatigue; quantify the additional incident fatigue caused by COVID-19; assess factors associated with incident fatigue; and describe deaths and hospitalizations among patients with incident fatigue after SARS-CoV-2 infection.
This study was designed as a retrospective cohort analysis. We analyzed electronic health records (EHR) data collected from the University of Washington (UW) that included 3 hospitals (Harborview Medical Center, UW Medical Center Northwest, and UW Medical Center Montlake) and >300 primary care and specialty clinics providing healthcare services across the state of Washington, USA.
Case and Control Classification
COVID-19 patients consisted of adults (>18 years of age) having either a positive PCR test result for SARS-CoV-2 or a clinical diagnosis of COVID-19 during February 2020–February 2021 (9). A clinical diagnosis of COVID-19 was defined by an International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM), diagnostic code of B97.29, other coronavirus as the cause of diseases classified elsewhere; or U07.1, COVID-19, recorded in the EHR during February 2020–February 2021 (10). The index date was defined as the date of the first positive PCR result or the first clinical diagnosis, whichever was earlier.
Non–COVID-19 control patients were defined as adults who did not belong to the COVID-19 group and had >1 negative PCR for SARS-CoV-2 during February 2020–February 2021. The first negative test date is referred to as the index date. We excluded from this group persons with suspected COVID-19 or evidence of past COVID-19, including persons with any of the following ICD-10-CM codes: B34.2, coronavirus infection, unspecified; J12.82, pneumonia due to COVID-19; Z86.16, personal history of COVID-19; U09.9, post COVID-19 condition. We also excluded persons with a positive result on SARS-CoV-2 IgG.
Inclusion and Exclusion Criteria
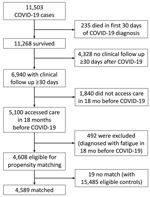
Patients in both COVID-19 case and non–COVID-19 control groups were required to survive the first 30 days from index date; access care >1 time on or after the day 30 from the index date, defined by having a diagnosis code or a laboratory test; access care >1 time during the 18 months before the index date for evaluation of preexisting fatigue diagnoses; and not be diagnosed with any codes used to define fatigue during the 18 months before the index date. During February 2020–February 2021, a total of 11,503 unique patients received a COVID-19 diagnosis. A total of 4,608 COVID-19 patients were eligible for matching (Figure 1).
We extracted data from 15,834 non–COVID-19 patients by querying the study database using the previously described inclusion and exclusion criteria, as well as the same requirements for accessing care. After data cleaning, 15,485 non–COVID-19 patients were determined to be eligible for matching.
Propensity Score Matching
We used propensity score matching to achieve balance in selected characteristics for COVID-19 and non–COVID-19 groups (11). We estimated propensity score using logistic regression with 22 input variables of age, sex, race, ethnicity, and whether the person had comorbidities derived from the Charlson Comorbidity Index (CCI) during the 18-month period before the index date (Table 1) (12). We then matched patients on the logit of propensity score using the greedy method with a caliper of 0.2 SD of the logit of the score.
Among 4,608 patients with COVID-19 who were eligible for matching, 19 (0.4%) had no matched controls. Among 4,589 patients with COVID-19 who had >1 match with 9,022 non–COVID-19 controls, 4,433 patients (96.6%) had 2 matched controls and 156 (3.4%) had 1. After matching, the standardized differences for 22 input variables used for estimating propensity score and for the index date were all <0.1, indicating between-group balances in these variables (13).
Outcome Measures
Outcome events of interest were patients with >1 diagnostic codes for fatigue or chronic fatigue recorded in the EHR during the postacute period. The postacute period was defined as the time between the 30th day since the index date and the last follow-up date up to January 2022.
Fatigue was defined by any of the following ICD-10-CM or International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), diagnostic codes recorded in EHR during the postacute period: G93.3, postviral fatigue syndrome; R53.82, chronic fatigue, unspecified; R53.83, other fatigue; 780.71, chronic fatigue syndrome/postviral fatigue syndrome; or 780.79, malaise and fatigue. We defined incident fatigue as a patient who had >1 diagnostic code for fatigue during the postacute period.
In this study, chronic fatigue is a subset of fatigue, defined as having any of the following 3 ICD-10-CM or ICD-9-CM codes recorded in the EHR during the postacute period: G93.3, postviral fatigue syndrome; R53.82, chronic fatigue, unspecified; and 780.71, chronic fatigue syndrome/postviral fatigue syndrome. We defined incident chronic fatigue as a patient who had >1 diagnostic code for chronic fatigue during the postacute period.
Follow-Up Time and Censoring
The last follow-up date was defined as the death date or the last date of having a clinical diagnosis or laboratory test up to January 2022. The follow-up time was calculated as time from the index date to the date of the first incident event for patients with an event, or as time from the index date to the last follow-up date for those without an event (right censoring).
Statistical Methods
We estimated incidence rates of fatigue and chronic fatigue for COVID-19 case and non–COVID-19 control groups using frequencies of events during the follow-up time, assuming a Poisson distribution of events. To quantify the attribution of COVID-19 to fatigue and chronic fatigue diagnoses, we used proportional hazards models that employed robust variance estimators to adjust for dependences associated with matching (14).
To examine potential predictors of incident fatigue among 4,589 patients with COVID-19, we used the Clinical Classifications Software Refined to aggregate diseases and conditions diagnosed within 18 months before COVID-19 into clinically meaningful categories (15). We analyzed data for categories with prevalence >1% and used the log-rank test to compare survival functions for each of the categories. We used multivariable proportional hazards models to identify factors associated with incident fatigue, adjusting for age, sex, and total number of comorbidities derived from the CCI (12,16). To assess the assumption of proportional hazards, we generated time-dependent covariates as a function of the predictors and follow-up time then evaluated the covariates in the model. We used proportions and crude relative risk (RR) to compare proportions of deaths and hospitalizations among patients with COVID-19 with fatigue versus those without fatigue. We performed all analyses using SAS 9.4 (SAS Institute, Inc., https://www.sas.com).
Human Subjects Considerations
This analysis is part of Project RELIEF (Research on COVID-19 Long-Term Effects). This activity was reviewed by the Centers for Disease Control and Prevention and was conducted consistent with applicable federal law and center policy. All protocols, procedures, and consent processes used in Project RELIEF were reviewed and approved by the University of Washington Institutional Review Board Committee A (STUDY00014595).
Patients
The study population had a mean age of 49.5 years for cases and 49.0 years for controls (Table 1). Approximately half of the patients were women. The most common comorbidities were diabetes and chronic obstructive pulmonary disease, each with 14% prevalence. Approximately 55% of the population had no comorbidities, and 6% had 4–10 comorbidities derived from the CCI.
Fatigue
During the total of 4,241.9 person-years of follow-up of 4,589 COVID-19 cases (median 11.4 months, range 1–21.4 months), 434 (9.5%) incident fatigue cases were identified, resulting in an incidence rate of 10.2/100 person-years. Of the 434 case-patients, 241 (55.5%) were women, the mean age was 52.6 (SD 17.3) years, and 165 (38.0%) patients did not have comorbidities.
The incidence rate of fatigue diagnosis was higher among women than among men and increased with advancing age (Table 2). We noted no strong evidence of a racial or ethnic difference in incidence of fatigue, except a slightly lower incidence among Black patients. Persons with more comorbidities experienced higher incidence rates than did persons without comorbidities. However, even among younger persons (18–29 years of age), those without comorbidities, and those who were not hospitalized for acute COVID-19, the incidence of fatigue was only slightly reduced (7.3/100 person-years for younger persons, 7.4/100 person-years for persons without comorbidities, and 9.9/100 person-years for persons who were not hospitalized).
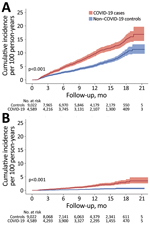
During the total of 7,939.1 person-years of follow-up of 9,022 non–COVID-19 controls (median 11.5 months, range 1–21.5 months), we identified 477 incident fatigue cases, resulting in an incidence rate of 6.0/100 person-years. The risk of incident fatigue was 68% higher among COVID-19 cases than among non–COVID-19 controls (hazard ratio 1.68, 95% CI 1.48–1.92; p<0.001) (Figure 2, panel A).
Chronic Fatigue
We next examined the incidence of chronic fatigue diagnosis, a subset of fatigue. During follow-up, 81 COVID-19 patients received a diagnosis of incident chronic fatigue, resulting in an incidence rate of 1.82 (95% CI 1.47–2.27)/100 person-years. The incidence rate of chronic fatigue among non–COVID-19 controls was 0.42 (95% CI 0.29–0.58)/100 person-years. The risk of developing chronic fatigue was significantly higher for COVID-19 cases compared with non–COVID-19 controls (HR 4.32, 95% CI 2.90–6.43; p<0.001). The difference between cumulative incidence for COVID-19 patients and non–COVID-19 controls continued to increase without apparent plateau >12 months after the index date (Figure 2, panel B).
Predictors of Incident Fatigue
Women were 39% more likely to have a fatigue diagnosis than men were after adjusting for age group and comorbidities (Table 2). Persons of advancing age groups were more likely than young adults 18–29 years of age to have a fatigue diagnosis in an unadjusted model. After adjusting for sex and comorbidities, the HRs for advancing age groups were still elevated, but the differences were no longer statistically significant. Those with comorbidities were significantly more likely to have incident fatigue compared with those with no comorbidities.
Among 36 diseases and conditions diagnosed in the 18 months before COVID-19 with a prevalence ≥1% that show difference in incident fatigue (log-rank p<0.05), 21 conditions remained associated (p<0.05) with incident fatigue when each was included in a multivariable proportional hazards model that adjusted for age, sex, and number of comorbidities. Obesity was associated with incident fatigue in the simple model, but the association became nonsignificant in the adjusted model. The risk for incident fatigue that was significantly higher for other diseases and conditions (Table 3) ranged from 27% increased risk for persons with hypertension to 93% increased risk for persons with gastritis and duodenitis.
Deaths and Hospitalizations
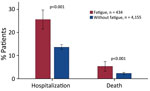
Patients with COVID-19 in whom incident fatigue developed had far worse clinical outcomes, as evidenced by deaths and hospitalizations, than patients without fatigue (Figure 3). Among 434 COVID-19 patients in whom fatigue developed, 111 (25.6%) were hospitalized >1 time during the postacute period, whereas 13.6% of 4,155 patients without incident fatigue were hospitalized (RR 1.88, 95% CI 1.57–2.24; p<0.001). Moreover, COVID-19 patients with incident fatigue were at higher risk of dying (23/434, 5.3%) during the postacute period than were COVID-19 patients without incident fatigue (94/4,155 [2.3%]; RR 2.34, 95% CI 1.50–3.66; p<0.001).
In this community-based cohort study of >4,500 adults followed for an average of 11.4 months after COVID-19 infection, fatigue developed in 9%. Even among persons not hospitalized for acute COVID-19 or those without comorbidities, the incidence of post–COVID-19 fatigue approached 10% per year. COVID-19 patients had 1.68 times the risk for fatigue in the follow-up period compared with concurrent, matched non–COVID-19 controls. The risk for chronic fatigue was even more marked: patients with COVID-19 had 4.32 times the risk for chronic fatigue than did controls.
This study provides new estimates of the incidence rate of fatigue using person-years of follow-up of at-risk patients after COVID-19 infection. Our data can be put in the context of previous reports. A retrospective study of EHR data reported 12.8% of patients had received a diagnosis of incident fatigue within 6 months of COVID-19 infection (17). That report had a different follow-up time and did not describe whether preexisting fatigue cases were excluded from the incident fatigue counts, which might explain their higher proportion than our estimate of 9.5%. In another retrospective study of insurance claims where preexisting fatigue diagnoses were excluded from the incident event count, 4.6% of COVID-19 patients received a diagnosis of fatigue during the follow-up of <6 months (18). That proportion approaches our estimate of 5% cumulative incidence of fatigue for 6 months.
An incidence rate of 4.2/100 person-years for post–COVID-19 fatigue was reported from Germany (19). That study counted cases occurring from 3 months after infection, which potentially contributed to lower event counts. Of note, follow-up times for patients with an incident event were assigned on the basis of the calendar quarter of the insurance claim submission, and the follow-up times for patients without events were not described. A combination of those methodological differences might have contributed to the lower incidence estimate in that study.
The excess risk for fatigue attributable to COVID-19 estimated in our study is in range of previous estimates. Specifically, our hazard ratio for fatigue of 1.68 (95% CI 1.48–1.92) indicates that when compared with a concurrent control population without COVID-19, COVID-19 contributes to a 68% increase in the rate of incident fatigue. This finding mirrors the previous estimates in studies using EHR data (HR 1.65) or administrative claims data (HR 2.20, 95% CI 1.48–3.27) in the United States or in Germany (incidence rate ratio [IRR] 1.97, 95% CI 1.89–2.06) (17–19).
This study also provides new estimates of incidence rate of chronic fatigue, including ME/CFS after COVID-19 illness. The incidence rate of 1.8/100 person-years is notable, as is the observation that chronic fatigue diagnoses continued in the 18 months of follow-up after COVID-19 detection. The extended period of incident chronic fatigue occurrences suggests a persistent effect but could also indicate a delay in diagnosing fatigue as a separate symptom or diagnosis. The hazard ratio for chronic fatigue (4.32, 95% CI 2.90–6.43) indicates that COVID-19 illness results in 4.3 times the risk for chronic fatigue compared with non–COVID-19 group. That increase is similar to findings from a study of chronic fatigue syndrome in Germany (IRR 3.04, 95% CI 2.66–3.48) (19). Although chronic fatigue is not the same as chronic fatigue syndrome or ME/CFS, which requires additional symptoms for diagnosis, including activity limitation, postexertional malaise, unrefreshing sleep, and either cognitive impairment or orthostatic intolerance (20), the ICD-9 and ICD-10 codes used for the diagnosis of ME/CFS were included in the diagnostic codes used to define the chronic fatigue diagnosis. The recently implemented diagnostic code G93.32 for ME/CFS when used in conjunction with code U09.9, post COVID-19 condition, will be instrumental in identifying COVID-19–related ME/CFS in future research (21).
We found many diseases and conditions to be associated with post–COVID-19 fatigue. Those associations might provide useful prognostic information for the assessment of patients with COVID-19. Patients with mood disorders were previously reported to be at higher risk for illness and death during acute COVID-19 and increased risk of needing postacute care (22). Our findings indicate that patients with a history of mood disorders are also at increased risk for post–COVID-19 fatigue. The association of post–COVID-19 fatigue with pain syndromes and sleep disorders is supported by previous research in non–COVID-19 populations (23).
Our study has several strengths, including addressing a critical data gap in incidence measure of post–COVID-19 fatigue; robust application of cohort methodologies in incidence estimation using EHR data; that the EHR data were collected from a comprehensive, multiclinic, multihospital health system; a well-defined population at risk for identifying the incident event; and the rigorous selection of concurrent non–COVID-19 matched controls. However, several limitations deserve consideration. First, because we used EHR data for this study, our findings apply only to patients who access care. Future studies are needed to understand the incidence of post-COVID fatigue among those who do not access care, which would likely require different methods. Second, data on exact date of onset, duration, and severity of fatigue or related functional limitations are unavailable for further characterization. The date of fatigue documented in EHR does not necessarily represent the date of symptom onset. In addition, providers might continue to document fatigue or carry forward the diagnosis. Therefore, relying on coding for chronic fatigue without an exact date of symptom onset might underestimate incidence of chronic fatigue. Moreover, the sense of fatigue is subjective and can be underrecorded if it is being considered as part of a disease process. The introduction of code U09.9, post COVID-19 condition, in October 2021 would not change results because it would need to be coded in conjunction with fatigue. Third, data on COVID-19 vaccination were not recorded for most patients, precluding further analysis. Fourth, the relatively small number of patients with fatigue who experienced hospitalization or death during follow-up precluded further multivariable analyses to adjust for potential confounders. The unadjusted association between fatigue and hospitalization or death might have been the result of the greater comorbidities seen in persons with fatigue. Fifth, this article is focused on post–COVID-19 fatigue, but PCC is generally experienced with multisystem symptom clusters. This study was not designed to capture symptom clusters, such as postexertional malaise or symptoms other than fatigue that might also be associated with subsequent outcomes. Last, our data were limited to persons who were tested or received a diagnosis in the first 13 months of the pandemic in Washington, which was 3 months before the Delta variant was detected and 9 months before Omicron was detected (24). Early research indicates that the prevalence of post–COVID-19 fatigue was similar across pre-Delta variants, Delta variants, and Omicron variants, but the prevalence of severe fatigue after infections with pre-Delta variants was slightly higher than for other variants (25). Future research is needed to estimate incidence rates of fatigue after infections with Delta and Omicron variants and compare them with the findings from this study.
In our unadjusted analyses, patients with COVID-19 who had incident fatigue were at higher risk for hospitalization and death than were persons without incident fatigue. The severe outcome is likely driven, at least in part, by some of the comorbidities and predictors identified in this study. Elevated death rate was previously reported among fatigued patients without COVID-19 (HR 1.45) (26). Increased awareness of fatigue and other PCC is warranted to enable patients to seek early care when needed. Further research is also warranted to investigate the causes and preventive measures for the severe outcomes associated with post-COVID fatigue.
In conclusion, our data indicate that COVID-19 is associated with a significant increase in new fatigue diagnoses, and physicians should be aware that fatigue might occur or be newly recognized >1 year after acute COVID-19. Future study is needed to better understand the possible association between fatigue and clinical outcomes. The high incidence rates of fatigue reinforce the need for public health actions to prevent infections, to provide clinical care to those in need, and to find effective treatments for post–acute COVID-19 fatigue.
Dr. Vu is an epidemiologist in the Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention. His primary research interests are infectious diseases and postinfectious sequelae.
Acknowledgment
This work was supported by a contract with the Centers for Disease Control and Prevention (contract no. 75D30121C10207).
References
- Centers for Disease Control and Prevention. Long COVID—household pulse survey [cited 2023 Feb 6]. https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm
- Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–5. DOIPubMedGoogle Scholar
- O’Mahoney LL, Routen A, Gillies C, Ekezie W, Welford A, Zhang A, et al. The prevalence and long-term health effects of Long Covid among hospitalised and non-hospitalised populations: A systematic review and meta-analysis. EClinicalMedicine. 2022;55:
101762 . DOIPubMedGoogle Scholar - Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al.; RECOVER Consortium. RECOVER Consortium. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–46. DOIPubMedGoogle Scholar
- Bateman L, Bested AC, Bonilla HF, Chheda BV, Chu L, Curtin JM, et al. Myalgic Encephalomyelitis/chronic fatigue syndrome: essentials of diagnosis and management. Mayo Clin Proc. 2021;96:2861–78. DOIPubMedGoogle Scholar
- Choutka J, Jansari V, Hornig M, Iwasaki A. Unexplained post-acute infection syndromes. Nat Med. 2022;28:911–23. DOIPubMedGoogle Scholar
- Horberg MA, Watson E, Bhatia M, Jefferson C, Certa JM, Kim S, et al. Post-acute sequelae of SARS-CoV-2 with clinical condition definitions and comparison in a matched cohort. Nat Commun. 2022;13:5822. DOIPubMedGoogle Scholar
- Wisk LE, Gottlieb MA, Spatz ES, Yu H, Wang RC, Slovis BH, et al.; INSPIRE Group. INSPIRE Group. Association of initial SARS-CoV-2 test positivity with patient-reported well-being 3 months after a symptomatic illness. JAMA Netw Open. 2022;5:
e2244486 . DOIPubMedGoogle Scholar - Centers for Disease Control and Prevention. ICD-10-CM official coding and reporting guidelines: April 1, 2020 through September 30, 2020 [cited 2023 Aug 25]. https://www.cdc.gov/nchs/data/icd/covid-19-guidelines-final.pdf
- Centers for Disease Control and Prevention. Co-occurrence of other respiratory illnesses for hospital confirmed COVID-19 encounters by week from selected hospitals [cited 2023 Mar 10]. https://www.cdc.gov/nchs/covid19/nhcs/other-respiratory-illnesses.htm
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. DOIGoogle Scholar
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. DOIPubMedGoogle Scholar
- Zhang Z, Kim HJ, Lonjon G, Zhu Y; written on behalf of AME Big-Data Clinical Trial Collaborative Group. Balance diagnostics after propensity score matching. Ann Transl Med. 2019;7:16. DOIPubMedGoogle Scholar
- Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–8. DOIGoogle Scholar
- Agency for Healthcare Research and Quality. Clinical Classifications Software Refined (CCSR) [cited 2023 Jan 19]. https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp
- Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82. DOIPubMedGoogle Scholar
- Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: A 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18:
e1003773 . DOIPubMedGoogle Scholar - Daugherty SE, Guo Y, Heath K, Dasmariñas MC, Jubilo KG, Samranvedhya J, et al. Risk of clinical sequelae after the acute phase of SARS-CoV-2 infection: retrospective cohort study. BMJ. 2021;373:n1098. DOIPubMedGoogle Scholar
- Roessler M, Tesch F, Batram M, Jacob J, Loser F, Weidinger O, et al. Post-COVID-19-associated morbidity in children, adolescents, and adults: A matched cohort study including more than 157,000 individuals with COVID-19 in Germany. PLoS Med. 2022;19:
e1004122 . DOIPubMedGoogle Scholar - Institute of Medicine. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: redefining an illness. Washington: The National Academies; 2015.
- Centers for Disease Control and Prevention. Myalgic encephalomyelitis/chronic fatigue syndrome: ICD-10-CM codes [cited 2023 Dec 15]. https://www.cdc.gov/me-cfs/healthcare-providers/diagnosis/icd-10.html
- Castro VM, Gunning FM, McCoy TH, Perlis RH. Mood disorders and outcomes of COVID-19 hospitalizations. Am J Psychiatry. 2021;178:541–7. DOIPubMedGoogle Scholar
- Thomas KS, Motivala S, Olmstead R, Irwin MR. Sleep depth and fatigue: role of cellular inflammatory activation. Brain Behav Immun. 2011;25:53–8. DOIPubMedGoogle Scholar
- Washington State Department of Health. SARS-CoV-2 sequencing and variants in Washington state [cited 2023 May 5]. https://doh.wa.gov/sites/default/files/2022-02/420-316-SequencingAndVariantsReport.pdf
- Gottlieb M, Wang RC, Yu H, Spatz ES, Montoy JCC, Rodriguez RM, et al.; Innovative Support for Patients with SARS-CoV-2 Infections Registry (INSPIRE) Group. Severe fatigue and persistent symptoms at 3 months following severe acute respiratory syndrome coronavirus 2 infections during the pre-Delta, Delta, and Omicron time periods: a multicenter prospective cohort study. Clin Infect Dis. 2023;76:1930–41. DOIPubMedGoogle Scholar
- Goklemez S, Saligan LN, Pirsl F, Holtzman NG, Ostojic A, Steinberg SM, et al. Clinical characterization and cytokine profile of fatigue in hematologic malignancy patients with chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56:2934–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: February 14, 2024
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Quan M. Vu, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H24-12, Atlanta, GA 30329-4018, USA
Top