Volume 30, Number 3—March 2024
Research Letter
Detection of Invasive Anopheles stephensi Mosquitoes through Molecular Surveillance, Ghana
Abstract
The invasive Anopheles stephensi mosquito has rapidly expanded in range in Africa over the past decade. Consistent with World Health Organization guidelines, routine entomologic surveillance of malaria vectors in Accra, Ghana, now includes morphologic and molecular surveillance of An. stephensi mosquitoes. We report detection of An. stephensi mosquitoes in Ghana.
Anopheles stephensi is an invasive mosquito species originating from parts of Southeast Asia and the Arabian Peninsula (1). Over the past decade, An. stephensi mosquitoes have been expanding in range and have now been documented in several countries in Africa (2). First detected in Djibouti, on the Horn of Africa, in 2012, this vector has been implicated in urban malaria outbreaks (3). They were also detected in Ethiopia in 2016 and 2018 (4,5). An. stephensi mosquitoes were subsequently detected in Sudan (2016), Somalia (2019), Nigeria (2020), and Kenya (2023) (2,3,5–7). This invasive vector poses a major threat to current malaria control and elimination efforts. The ability of An. stephensi mosquitoes to breed in artificial containers enables them to thrive in urban areas, setting them apart from other major malaria vectors (8). This species can also transmit both Plasmodium falciparum and P. vivax protozoa (1). Although malaria is widely a rural disease, transmission in urban areas may rise because of the establishment of An. stephensi mosquitoes, putting ≈126 million persons at risk of malaria (2,8). The World Health Organization issued an initiative in 2022 aimed at strengthening surveillance to help stop the spread of An. stephensi mosquitoes in sub-Saharan Africa (2). Morphologic and molecular surveillance of An. stephensi mosquitoes were incorporated into routine entomologic surveillance of malaria vectors in the city of Accra, Ghana, after the World Health Organization initiative (2). This study outlines the entomologic surveillance that documents the identification of this invasive species in Ghana.
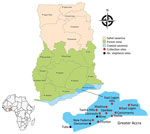
We conducted routine entomologic surveillance in 8 sites within the city of Accra, Ghana, during January 2022–July 2022 (Figure). We conducted larval sampling in all mosquito larval breeding habitats encountered in each of the sites. We recorded the total number of dips, larvae, and pupae, and we calculated the larval density as the ratio of the number of larvae collected per dip. We conducted larval sampling in the dry (February–March) and rainy (June–July) seasons of 2022. We transported larval samples to the insectary at the Department of Medical Microbiology, University of Ghana Medical School (Accra, Ghana), where we raised them into adults for morphologic and molecular species identification. We further identified members of the An. gambiae sensu lato, complex and sibling species by using PCR. We performed PCR amplifications to detect An. stephensi mosquitoes by using primers targeting the internal transcribed spacer region on the basis of on previously described protocols by Singh et al. (9). After PCR, were subjected 2 mosquitoes to Sanger sequencing of the internal transcribed spacer 2 regions and analyzed them on the basis of comparisons to the National Center for Biotechnology Information database.
We identified a total of 1,169 mosquitoes obtained from the larval sampling by using morphologic keys and PCR methods for speciation. Out of that number, 551 (47.13%) were An. gambiae sensu stricto, 582 (49.79%) An. coluzzii, and 32 (2.74%) hybrids of both species. We identified 4 samples (0.34%) as An. stephensi by using a modified PCR-based method by Singh et al. (9) and sequencing (Appendix Table 1). Results from BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) showed that the An. stephensi mosquito samples had 100% sequence similarity with An. stephensi voucher A268 5.8S ribosomal RNA gene and internal transcribed spacer 2 (GenBank accession no. MH650999.1) (Table).
We found An. stephensi mosquitoes in larval samples from urban areas of Accra, Ghana, specifically the suburbs of Tuba, Dansoman, and Nima. We found An. stephensi mosquitoes breeding in dugout wells within irrigated vegetable farms and roadside ditches (Appendix Figure), habitats that are distinct from the typical ones observed in Asia and East Africa (10). In addition, An. stephensi larvae were present alongside An. gambiae s.s. and An. coluzzii mosquitoes, even though An. stephensi larvae are usually present alongside Aedes mosquitoes.
The spread of An. stephensi mosquitoes in Africa is thought to have occurred through land borders, air travel, or seaports. However, we discovered the mosquitoes at considerable distances from those points of entry, suggesting possible earlier introductions. Expanding surveillance efforts for An. stephensi mosquitoes is crucial to curbing the dissemination of this invasive species within Ghana, which could potentially elevate malaria prevalence in the city of Accra, traditionally considered a low malaria transmission zone within Ghana.
This report of the invasion of An. stephensi mosquitoes in Accra, Ghana, represents a major public health concern, given the heightened risk of urban malaria outbreaks. It is imperative to reinforce surveillance and response strategies in both rural and urban settings across Ghana, with specific attention directed toward An. stephensi mosquitoes, to mitigate the spread of this invasive species.
Dr. Afrane is a professor of vector biology at the University of Ghana. His research focuses on vector and parasite biology and epidemiology.
Acknowledgments
We thank the Eck Institute for Global Health, University of Notre Dame, for support with sequencing.
This study was supported by grants from the National Institute of Health (NIH grant nos. R01 A1123074 and D43 TW 011513).
Author contributions: Y.A.A., K.M. ,and N.F.L. were responsible for the study design, supervised the data collection, and contributed to the writing of the manuscript. A.A., A.R.M., Y.A.B., C.M.O.-A., S.A.Y., and I.S. performed the data collection, laboratory work, and analysis. A.A., Y.A.A., and NFL drafted the manuscript. All the authors read and approved the final manuscript.
References
- Sinka ME, Bangs MJ, Manguin S, Chareonviriyaphap T, Patil AP, Temperley WH, et al. The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. DOIPubMedGoogle Scholar
- World Health Organization. WHO initiative to stop the spread of Anopheles stephensi in Africa. 2023 Aug 15 [cited 2023 Aug 22]. https://www.who.int/publications/i/item/WHO-UCN-GMP-2022.06
- Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop. 2014;139:39–43. DOIPubMedGoogle Scholar
- Balkew M, Mumba P, Dengela D, Yohannes G, Getachew D, Yared S, et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors. 2020;13:35. DOIPubMedGoogle Scholar
- Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop. 2018;188:180–6. DOIPubMedGoogle Scholar
- Ahmed A, Khogali R, Elnour MB, Nakao R, Salim B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasit Vectors. 2021;14:511. DOIPubMedGoogle Scholar
- Ochomo EO, Milanoi S. Molecular surveillance leads to the first detection of Anopheles stephensi in Kenya. 2023 [cited 2023 Sep 9]. https://www.researchsquare.com/article/rs-2498485/v1
- Sinka ME, Pironon S, Massey NC, Longbottom J, Hemingway J, Moyes CL, et al. A new malaria vector in Africa: Predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci U S A. 2020;117:24900–8. DOIPubMedGoogle Scholar
- Singh OP, Kaur T, Sharma G, Kona MP, Mishra S, Kapoor N, et al. Molecular tools for early detection of invasive malaria vector Anopheles stephensi mosquitoes. Emerg Infect Dis. 2023;29:36–44. DOIPubMedGoogle Scholar
- Thomas S, Ravishankaran S, Justin NA, Asokan A, Mathai MT, Valecha N, et al. Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malar J. 2017;16:111. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: February 05, 2024
Table of Contents – Volume 30, Number 3—March 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Yaw Asare Afrane, University of Ghana, Department of Medical Microbiology, University of Ghana Medical School, University of Ghana, PO Box KB 4236, Accra, Ghana
Top