Volume 30, Number 4—April 2024
Research Letter
Crimean-Congo Hemorrhagic Fever Virus Seroprevalence in Human and Livestock Populations, Northern Tanzania
Abstract
We conducted a cross-sectional study of Crimean-Congo hemorrhagic fever virus (CCHFV) in northern Tanzania. CCHFV seroprevalence in humans and ruminant livestock was high, as were spatial heterogeneity levels. CCHFV could represent an unrecognized human health risk in this region and should be included as a differential diagnosis for febrile illness.
Crimean-Congo hemorrhagic fever virus (CCHFV) is a tickborne orthonairovirus with potential to cause severe Crimean-Congo hemorrhagic fever (CCHF) disease in humans, which can lead to human-to-human transmission (1). CCHFV is a World Health Organization priority pathogen for research and development (2). Although a wide range of wild and domestic animals can be infected (3), CCHFV does not typically cause clinical disease in nonhuman species (1). In eastern Africa, intermittent outbreaks of CCHF disease in humans have occurred in Uganda since 2013 (4), but the epidemiology of CCHFV remains poorly understood. Northern Tanzania, neighboring Uganda, has been identified as an area likely to be at high risk for human disease caused by CCHFV, because competent tick vectors and suitable environmental conditions exist in the region (5), but no clinical CCHF cases have yet been reported in the country.
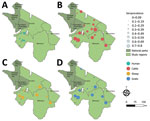
To investigate CCHFV exposure in northern Tanzania, we performed serologic testing on human and ruminant livestock serum samples collected in 2016 during an investigation of several zoonotic pathogens (6) (Appendix). The study used a multilevel sampling frame of 351 humans and 7,456 randomly selected livestock in linked households in Arusha and Manyara Regions (Figure). We tested serum samples by using the ID Screen CCHF Double Antigen Multi-species ELISA (IDvet, https://www.innovative-diagnostics.com) (Appendix). We estimated seroprevalence by using the Survey package in R (The R Foundation for Statistical Computing, https://www.r-project.org) (7). We assessed species-level differences in seroprevalence by using a mixed-effects model with household and village as random effects. We investigated patterns of spatial autocorrelation in village-level seroprevalence by using the Moran I statistic and assessed correlation of village-level seroprevalence between species pairs by using the Pearson correlation coefficient (ρ) (Appendix).
Overall, seroprevalence was high in all livestock species: cattle 49.6% (95% CI 40.0%–59.2%), goats 33.8% (95% CI 21.7%–47.5%), sheep 27.8% (95% CI 17.0%–40.6%) (Table; Figure). Sheep and goats had significantly lower odds of exposure than cattle: sheep OR was 0.32 (95% CI 0.27–0.37, p<0.001) and goats OR 0.45 (95% CI 0.39–0.51; p<0.001). Village-level seroprevalence ranged widely in all species but values were consistent with those reported elsewhere in East Africa (3) (Table). The finding of higher seroprevalence in cattle than in sheep and goats is also consistent with other settings in Africa (3) and might reflect differences in host feeding preferences of Hyalomma spp. ticks, considered chief vectors of CCHFV (1). However, further work is required to understand the relative contribution of different host species to viral maintenance, and their relationship to human infection risk.
Overall, human seroprevalence was 15.1% (95% CI 11.7%–19.2%), but village-level seroprevalence varied widely between study sites (Table). Seroprevalence was similar to that reported in health-care-seeking patients in Kenya in 2012 (8), but higher than the 1.2% seroprevalence reported in community participants elsewhere in Tanzania (9). However, interpretation of those regional comparisons is challenging in light of the substantial observed between-village variation in our study (Table).
Assessment of spatial autocorrelation via Moran I statistic showed no evidence of village-level spatial autocorrelation in livestock (Table), suggesting that although context-specific drivers, such as husbandry practices and local agroecology are likely involved, drivers of exposure were not observable at this scale. In contrast, we observed significant positive spatial autocorrelation in the village-level human seroprevalence (Moran I statistic 0.43; p<0.001) and clustering of higher seroprevalence villages in the western part of Manyara (Figure). In addition, species-pair correlations showed that village-level human and livestock seroprevalence were not well correlated (cattle, ρ = 0.34, p = 0.142; sheep, ρ = 0.35, p = 0.13; goats, ρ = 0.42, p = 0.062), and we saw high human seroprevalence in some low livestock seroprevalence locations and vice versa (Appendix). That heterogeneity, combined with differences in spatial distribution, could suggest different drivers of exposure in livestock and human populations. However, discrepancies in sample size could exaggerate those differences, so further linked investigation into human and livestock exposure and patterns of tick infection are required. Further exploration of specific risk factors is ongoing and could provide clarity on drivers of exposure.
The high human exposure levels to CCHFV implies that clinical CCHF is a potentially serious, underdiagnosed health risk in this population and suggests that CCHF should be included as a differential diagnosis for undifferentiated febrile illness in northern Tanzania. However, evidence of human seropositivity in the absence of clinical cases is common, even where health professionals are familiar with CCHF diagnosis (8,10). The causes of disease emergence in such populations are poorly understood, and further research into regions like northern Tanzania, where the virus is endemic but human disease has not been reported, is critical to understanding human disease risk.
In conclusion, we found that CCHFV is circulating widely in livestock across northern Tanzania. CCHFV seroprevalence in the region shows high spatial heterogeneity and further investigations are needed to understand drivers of exposure. In addition, high human seroprevalence demonstrates widespread exposure of the population to CCHFV and suggests that CCHF should be included as a differential diagnosis for febrile illness in this region.
Dr. Hughes is a veterinarian and post-doctoral researcher who works for the Global Burden of Animal Diseases Programme at the University of Liverpool, Liverpool, UK. Her research interests include emerging and endemic zoonoses, disease burden estimation, and One Health approaches to animal and human health.
Acknowledgment
This article was preprinted at https://www.medrxiv.org/content/10.1101/2023.08.31.23294720v1.
References
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–89. DOIPubMedGoogle Scholar
- World Health Organization. Prioritizing diseases for research and development in emergency contexts [cited 2022 Feb 26]. https://www.who.int/activities/prioritizing-diseases-for-research-and-development-in-emergency-contexts
- Spengler JR, Bergeron É, Rollin PE. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Negl Trop Dis. 2016;10:
e0004210 . DOIPubMedGoogle Scholar - Balinandi S, von Brömssen C, Tumusiime A, Kyondo J, Kwon H, Monteil VM, et al. Serological and molecular study of Crimean-Congo Hemorrhagic Fever Virus in cattle from selected districts in Uganda. J Virol Methods. 2021;290:114075–114075. DOIPubMedGoogle Scholar
- Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. 2015;109:503–13. DOIPubMedGoogle Scholar
- Herzog CM, de Glanville WA, Willett BJ, Kibona TJ, Cattadori IM, Kapur V, et al. Pastoral production is associated with increased peste des petits ruminants seroprevalence in northern Tanzania across sheep, goats and cattle. Epidemiol Infect. 2019;147:
e242 . DOIPubMedGoogle Scholar - Christova I, Panayotova E, Groschup MH, Trifonova I, Tchakarova S, Sas MA. High seroprevalence for Crimean-Congo haemorrhagic fever virus in ruminants in the absence of reported human cases in many regions of Bulgaria. Exp Appl Acarol. 2018;75:227–34. DOIPubMedGoogle Scholar
- Rugarabamu S, Mwanyika GO, Rumisha SF, Sindato C, Lim HY, Misinzo G, et al. Seroprevalence and associated risk factors of selected zoonotic viral hemorrhagic fevers in Tanzania. Int J Infect Dis. 2021;109:174–81. DOIPubMedGoogle Scholar
- Hoogstraal H. The pidemiology of tick-borne CCHF in Asia Europe and Africa. J Med Entomol. 1979;15:307–417. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 18, 2024
Table of Contents – Volume 30, Number 4—April 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ellen C. Hughes, c/o Prof. Brian Willett, MRC-University of Glasgow Centre for Virus Research, Garscube Campus, 464 Bearsden Rd, Glasgow, Scotland G61 1QH, UK
Top