Volume 30, Number 4—April 2024
Dispatch
Melioidosis in Patients with COVID-19 Exposed to Contaminated Tap Water, Thailand, 2021
Abstract
In September 2021, a total of 25 patients diagnosed with COVID-19 developed acute melioidosis after (median 7 days) admission to a COVID-19 field hospital in Thailand. Eight nonpotable tap water samples and 6 soil samples were culture-positive for Burkholderia pseudomallei. Genomic analysis suggested contaminated tap water as the likely cause of illness.
Melioidosis is an infectious disease caused by the gram-negative bacillus Burkholderia pseudomallei, which is commonly present in soil and water in tropical countries (1,2). Naturally acquired infections result from skin inoculation, inhalation, or ingestion of B. pseudomallei (1). In 2020 and 2021, multiple COVID-19 field hospitals were set up in Thailand for cases of mild and moderate COVID-19 infection. We report 25 cases of acute melioidosis among patients diagnosed with COVID-19 who were being managed at a COVID-19 field hospital in Saraburi Province, central Thailand. Our study received ethical approval from the Committee of the Faculty of Tropical Medicine, Mahidol University (TMEC 24-006).
On September 8, 2021, the Department of Disease Control, Ministry of Public Health, Thailand, was alerted to a cluster of 20 patients with culture-confirmed melioidosis (case nos. 1–20; Table). The 20 patients had been admitted to a single field hospital in Saraburi Province, which had been designated a treatment facility for COVID-19, and were transferred to Kaeng Khoi Hospital, Saraburi Province, because they developed fever or pneumonia. Previously, Saraburi Province had diagnosed ≈8–12 culture-confirmed melioidosis cases per year (2). The outbreak investigation team suspected that nonpotable tap water (NPTW) was the source of infection because there were no other apparent sources (Appendix). The initial response included the immediate transfer of patients with diabetes and those who had received steroid therapy for COVID-19 to other hospitals, followed by re-emphasizing to staff the recommended prevention strategies for melioidosis (3). The recommendations included avoiding direct exposure to soil and environmental water and drinking only boiled or bottled water.
Health officials immediately planned and conducted an environmental investigation. During September 10–16, 2021, the outbreak investigation team collected samples from the field hospital, including 8 commercially bottled drinking water (CBDW) samples (500 mL–1 L), 37 NPTW samples from 10 locations (1-L samples; 3–4 samples per location at different time points), and 50 soil samples (100 g per sample) (Appendix). We isolated B. pseudomallei from the environmental samples according to previously described methods (4,5). None of the CBDW samples, 6 (12%) of 50 soil samples, and 8 (22%) of 37 NPTW samples (from 4 locations) were culture-positive for B. pseudomallei. The median quantitative count of B. pseudomallei in NPTW was 24.5 CFU/L (range 4–58 CFU/L) and in soil was 82 CFU/g (range <1–119 CFU/g). The outbreak investigation team found that the chlorination system for NPTW was not well maintained. Patients reported that they drank only CBDW and never drank NPTW, which was used for other domestic purposes, such as brushing their teeth, rinsing their mouths, and showering. The chlorination system was successfully repaired, and chlorine levels were maintained >1 ppm beginning on September 10. A further 5 melioidosis cases were identified, all of whom had been admitted before September 10. No new melioidosis cases among those who had stayed at the field hospital were reported after September 16.
Of the 25 patients diagnosed with melioidosis (Table), 12 (48%) were female and 13 (51%) male; median age was 59 (interquartile range 56–62, range 34–73) years. All patients had received a diagnosis of COVID-19, confirmed by PCR during August 16–29, 2021, and had been admitted to the field hospital in August 22–September 2, 2021. A total of 15 (60%) patients had diabetes, and all 25 (100%) patients had received steroids as part of their COVID-19 treatment. The date range of onset of symptoms attributed to melioidosis was September 1–11. The median time from admission to the field hospital to the onset of the melioidosis symptoms was 7 (interquartile range 5–9, range 4–20) days.
The most common clinical manifestations of melioidosis among patients in this cluster were secondary bacterial pneumonia (n = 22 patients [88%]) and fever (n = 15 [60%]) (Table). Clinical specimens that were culture positive for B. pseudomallei were blood (n = 24 [96%]) and sputum (n = 1 [4%]). In-hospital mortality for patients we studied was 32% (8/25). Of the fatal cases, 3 patients (case nos. 2, 4, and 8) died without receiving ceftazidime or meropenem, which are recommended parenteral antibiotics for treatment of melioidosis. A total of 17 (68%) cases completed >10 days of parenteral ceftazidime or meropenem and subsequently received a course of oral eradicative treatment.
We confirmed the first 20 clinical isolates and all environmental isolates as B. pseudomallei at the Mahidol-Oxford Tropical Medicine Research Unit laboratory, Bangkok, by using a combination of colony morphology on Ashdown agar, latex agglutination test, arabinose assimilation test, and antimicrobial susceptibility tests. Testing revealed that all clinical and environmental isolates were susceptible to ceftazidime, meropenem, and trimethoprim/sulfamethoxazole. We performed whole-genome sequencing on 19 clinical isolates, 8 NPTW isolates, and 6 soil isolates (1 colony per patient or sample). We excluded 1 clinical isolate from analysis because of low sequencing depth of 7.5×. We deposited sequences in the European Nucleotide Archive (https://www.ebi.ac.uk/ena) (accession numbers in Appendix Table). We mapped isolates to the K96243 reference genome and used variant calls to construct a phylogeny after masking recombinant fragments, repetitive regions, and known B. pseudomallei genomic islands (6). We used genome assemblies to call multilocus sequence types (STs).
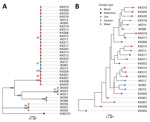
We categorized the isolates by both phylogenetic and multilocus sequence typing, and they clustered consistently into 4 groups. The largest cluster was ST689 and included all 19 blood and sputum samples, as well as 6 of 8 NPTW samples (Figure). The remaining soil and NPTW isolates formed 3 separate clusters (ST107, ST303, and ST315). Within the ST689 cluster, the isolates were closely related but not identical (12–98 SNP differences between isolates). The NPTW isolates were interspersed with clinical isolates in this cluster, suggesting that contaminated NPTW was a possible source of infection for these patients. Dating analysis was not feasible because of the absence of clock signals in the phylogeny.
Our study highlights that patients with viral infections (e.g., COVID-19) may be at risk for infection and death caused by melioidosis if exposed to NPTW contaminated by B. pseudomallei. Diabetes mellitus and conditions that impair innate and adaptive immune responses, particularly steroid use, are important risk factors for melioidosis (1). Diabetes mellitus is also a risk factor for COVID-19, and steroid treatment is recommended for patients with COVID-19 pneumonia (7). Therefore, unsurprisingly, co-infections with COVID-19 and B. pseudomallei have been reported occasionally (8,9; D. Chit Yee et al., unpub. data, https://wellcomeopenresearch.org/articles/7-160), including 1 of the 4 patients detected during the multistate outbreak of melioidosis caused by an imported aromatherapy spray in the United States (10), and now this cluster. Previous reports of co-infection with influenza A (11,12) or COVID-19 (9) and B. pseudomallei suggested that melioidosis could be reactivated from a latent focus following viral infection. However, the timeline of the cluster, the identified source, and genomic analysis suggest that the patients in this cluster represented recently acquired secondary infections after COVID-19. The route of infection in this cluster was probably skin exposure to contaminated NPTW at a high-infecting dose, although ingestion or inhalation are also possible.
An unknown proportion of melioidosis patients in melioidosis-endemic areas could be related to exposure to contaminated NPTW. More studies on the effects of B. pseudomallei-contaminated NPTW and its disinfection (13) in melioidosis endemic areas are required. Because general recommendations for melioidosis prevention (3) do not emphasize the disinfection of NPTW, those recommendations may be inadequate and should be revisited.
Dr. Tantirat is assistant director of Information Technology at Saraburi Hospital and serves as the deputy director of the Bureau of Digital Health within the Office of the Permanent Secretary at the Ministry of Public Health, Thailand. His key interests include epidemiology and disease control.
Acknowledgments
We thank all patients involved in providing information. We thank outbreak investigation team and all staff at Saraburi Hospital and Kaeng Khoi Hospital for their support.
This study was supported by the Department of Disease Control, Ministry of Public Health, Thailand, and the Wellcome Trust (220211/A/20/Z). This publication made use of the PubMLST website (https://pubmlst.org), developed by Keith Jolley and sited at the University of Oxford. The development of that website was funded by the Wellcome Trust. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of manuscript.
References
- Meumann EM, Limmathurotsakul D, Dunachie SJ, Wiersinga WJ, Currie BJ. Burkholderia pseudomallei and melioidosis. Nat Rev Microbiol. 2024;22:155–69. DOIPubMedGoogle Scholar
- Hantrakun V, Kongyu S, Klaytong P, Rongsumlee S, Day NPJ, Peacock SJ, et al. Clinical epidemiology of 7126 melioidosis patients in Thailand and the implications for a national notifiable diseases surveillance system. Open Forum Infect Dis. 2019;6:
ofz498 . DOIPubMedGoogle Scholar - Suntornsut P, Wongsuwan N, Malasit M, Kitphati R, Michie S, Peacock SJ, et al. Barriers and recommended interventions to prevent melioidosis in northeast Thailand: A focus group study using the behaviour change wheel. PLoS Negl Trop Dis. 2016;10:
e0004823 . DOIPubMedGoogle Scholar - Limmathurotsakul D, Wongsuvan G, Aanensen D, Ngamwilai S, Saiprom N, Rongkard P, et al. Melioidosis caused by Burkholderia pseudomallei in drinking water, Thailand, 2012. Emerg Infect Dis. 2014;20:265–8. DOIPubMedGoogle Scholar
- Hantrakun V, Rongkard P, Oyuchua M, Amornchai P, Lim C, Wuthiekanun V, et al. Soil nutrient depletion is associated with the presence of Burkholderia pseudomallei. Appl Environ Microbiol. 2016;82:7086–92. DOIPubMedGoogle Scholar
- Croucher NJ, Page AJ, Connor TR, Delaney AJ, Keane JA, Bentley SD, et al. Rapid phylogenetic analysis of large samples of recombinant bacterial whole genome sequences using Gubbins. Nucleic Acids Res. 2015;43:
e15 . DOIPubMedGoogle Scholar - Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a Clinician’s perspective. Diabetes Metab Syndr. 2020;14:971–8. DOIPubMedGoogle Scholar
- Gupta A, Siddiqui F, Purwar S, Joshi R, Mukhopadhyay C. Is it always COVID-19 in acute febrile illness in the tropics during the pandemic? PLoS Negl Trop Dis. 2022;16:
e0010891 . DOIPubMedGoogle Scholar - Gulati U, Nanduri AC, Juneja P, Kaufman D, Elrod MG, Kolton CB, et al. Case Report: A fatal case of latent melioidosis activated by COVID-19. Am J Trop Med Hyg. 2022;106:1170–2. DOIPubMedGoogle Scholar
- Gee JE, Bower WA, Kunkel A, Petras J, Gettings J, Bye M, et al. Multistate outbreak of melioidosis associated with imported aromatherapy spray. N Engl J Med. 2022;386:861–8. DOIPubMedGoogle Scholar
- Mackowiak PA, Smith JW. Septicemic melioidosis. Occurrence following acute influenza A six years after exposure in Vietnam. JAMA. 1978;240:764–6. DOIPubMedGoogle Scholar
- Tan WF, Lee HG. Concurrent influenza A and pulmonary melioidosis in pregnancy. Med J Malaysia. 2021;76:245–7.PubMedGoogle Scholar
- McRobb E, Kaestli M, Mayo M, Price EP, Sarovich DS, Godoy D, et al. Melioidosis from contaminated bore water and successful UV sterilization. Am J Trop Med Hyg. 2013;89:367–8. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 18, 2024
Table of Contents – Volume 30, Number 4—April 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Direk Limmathurotsakul, Mahidol-Oxford Tropical Medicine Research Unit, Faculty of Tropical Medicine, Mahidol University, 420/6 Ratchawithi Rd., Bangkok, 10400, Thailand
Top