Volume 30, Number 8—August 2024
Research
Potential of Pan-Tuberculosis Treatment to Drive Emergence of Novel Resistance
Figure 3
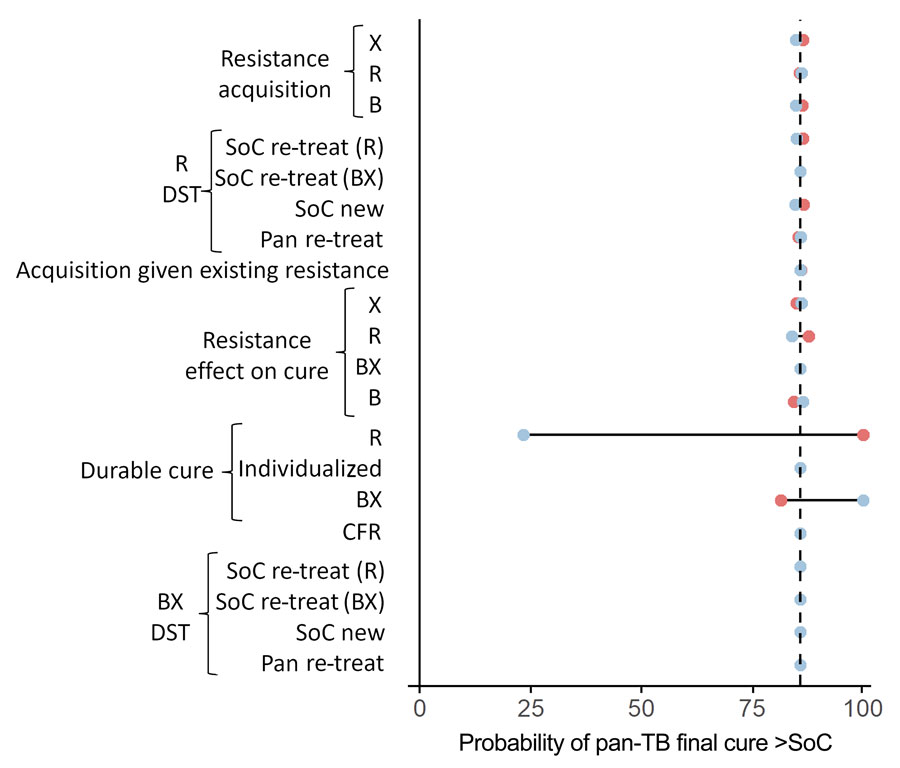
Figure 3. Univariate sensitivity analysis for initial TB treatment regimen comparison in study of potential of pan-TB treatment to drive emergence of novel resistance. Parameter sets are sampled with 1 parameter fixed at the extremes of its 95% CI. The outcome is the proportion of samples that result in more patients durably cured in the pan-TB scenario than the SoC scenario, within the first cohort of patients treated, and at current prevalence of resistance. Blue circles indicate use of upper bound of the parameter’s 95% CI; red circles indicate the lower bound. B, diarylquinolines; BX, diarylquinoline- and novel drug X–containing regimen; CFR, case-fatality ratio; DST, drug-susceptibility testing; R, rifamycins; re-treat, patients with previously treated TB; SoC, standard of care; X, additional novel drug X.