Volume 5, Number 5—October 1999
Synopsis
Morphologic and Molecular Characterization of New Cyclospora Species from Ethiopian Monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n.
Figure
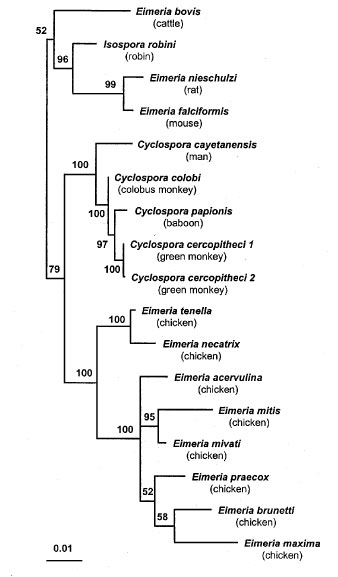
Figure. Phylogenetic tree for small subunit ribosomal RNA sequences of Cyclospora and Eimeria species. Quartet puzzling maximum likelihood results are shown, with Toxoplasma gondii as the outgroup. After analysis, the outgroup branch was removed for clarity. Numbers to the left of the nodes indicate the quartet puzzling support for each internal branch. The scale bar indicates an evolutionary distance of 0.01 nucleotides per position in the sequence. Vertical distances are for clarity only. GenBank accession numbers of the sequences used for analysis: Cyclospora cayetanensis, AF111183; C. cercopitheci 1, AF111184; C. cercopitheci 2, AF111185; C. colobi, AF111186; C. papionis, AF111187; Eimeria acervulina, U67115; E. bovis, U77084; E. brunetti, U67116; E. falciformis, AF080614; E. maxima, U67117; E. mitis, U40262; E. mivati, U76748; E. necatrix, U67119; E. nieschulzi, U40263; E. praecox, U67120; E. tenella, U40264; Isospora robini, AF080612; and Toxoplasma gondii, U12138. The sequences were aligned with the program CLUSTALW (20). Phylogenetic analysis was done with the maximum likelihood method-based PUZZLE program (21), as well as with the parsimony method-based PAUP program (22). Unreliably aligned regions were removed, and the final length of the alignment was 1692 columns. Aligned sequences are available from the authors upon request. 1Initially, we used a nested PCR protocol using primers CYCF1E and CYCR2B for the first step of the amplification and primers CYCF3E and CYCR4B for the second step (11). Samples were also amplified by using sets of PCR primers designed on the basis of the primers described above, but with the restriction sites removed. The primer CYCF1 (5'-ATTACCCAATGAA AACAGTTT- 3') was used in pairs with the primer CYCR4 (5'-TCGTCTTCAAACCCCCTACTG-3') to generate a DNA fragment of 577 bp. The other pair of primers, CYCF3 (5'-GCCTTCCGCGCTTCGCTGCGT-3') and CYCR2 (5'-TGC AGGAGAAGCCAAGGTAGG-3') was used to generate a fragment of 283 bp. To generate fragments spanning the full length of the SSU-rRNA coding region, we used generic apicomplexan PCR primer CRYPTOF (5'-AACCTG GTTGATCCTGCCAGT-3'), specific for the 5' end of the SSU-rRNA molecule and the apicomplexan generic PCR primer CRYPTOR (5'-GCTTGATCCTTCTGCAGGTTCACC TAC-3'), specific for the 3' end of this molecule. These generic primers were combined with the Cyclospora-specific primers (CYC-series, see above) to amplify overlapping fragments spanning the whole SSU-rRNA molecule. PCR products were analyzed by electrophoresis on 2% SeaKem GTG agarose (Cat. No. 50074, FMC Bioproducts, Rockland, ME), stained with ethidium bromide and visualized on a UV transilluminator.
References
- Ortega YR, Gilman RH, Sterling CR. A new coccidian parasite (Apicomplexa: Eimeriidae) from humans. J Parasitol. 1994;80:625–9. DOIPubMedGoogle Scholar
- Koumans EH, Katz DJ, Malecki JM, Kumar S, Wahlquist SP, Arrowood MJ, An outbreak of cyclosporiasis in Florida in 1995: a harbinger of multistate outbreaks in 1996 and 1997. Am J Trop Med Hyg. 1998;59:235–42.PubMedGoogle Scholar
- Herwaldt BL, Ackers ML. the Cyclospora Working Group. An outbreak in 1996 of cyclosporiasis associated with imported raspberries. N Engl J Med. 1997;336:1548–56. DOIPubMedGoogle Scholar
- Relman DA, Schmidt TM, Gajadhar A, Sogin M, Cross J, Yoder K, Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. J Infect Dis. 1996;173:440–5.PubMedGoogle Scholar
- Pieniazek NJ, Herwaldt BL. Reevaluating the molecular taxonomy: is human-associated Cyclospora a mammalian Eimeria species? Emerg Infect Dis. 1997;3:381–3. DOIPubMedGoogle Scholar
- García-López HL, Rodríguez-Tovar LE, Medina de la Garza CE. Identification of Cyclospora in poultry. Emerg Infect Dis. 1996;2:356–7. DOIPubMedGoogle Scholar
- Zerpa R, Uchima N, Huicho L. Cyclospora cayetanensis associated with watery diarrhoea in Peruvian patients. Am J Trop Med Hyg. 1995;98:325–9.
- Yai LE, Bauab AR, Hirschfeld MP, de Oliveira ML, Damaceno JT. The first two cases of Cyclospora in dogs, Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 1997;39:177–9. DOIPubMedGoogle Scholar
- Smith HV, Paton CA, Girdwood RWA, Mtambo MMA. Cyclospora in non-human primates. Vet Rec. 1996;138:528.PubMedGoogle Scholar
- da Silva AJ, Bornay-Llinares FJ, Moura INS, Slemenda SB, Tuttle JL, Pieniazek NJ. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol Diagn. 1999;4:57–64. DOIPubMedGoogle Scholar
- Pieniazek NJ, Slemenda SB, da Silva AJ, Alfano EM, Arrowood AJ. PCR confirmation of infections with Cyclospora cayetanensis. Emerg Infect Dis. 1996;2:357–9. DOIPubMedGoogle Scholar
- Ford PL, Duszynski DW, McAllister CT. Coccidia (Apicomplexa) from heteromyid rodents in the southwestern United States, Baja California, and northern Mexico with three new species from Chaetodipus hispidus. J Parasitol. 1990;76:325–31. DOIPubMedGoogle Scholar
- Ford PL, Duszynski DW. Coccidian parasites (Apicomplexa: Eimeriidae) from insectivores. VII. Six new species from the hairy-tailed mole, Parascalops breweri. J Parasitol. 1989;75:508–13. DOIPubMedGoogle Scholar
- Ortega YR, Nagle R, Gilman RH, Watanabe J, Miyagui J, Quispe H, Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. J Infect Dis. 1997;176:1584–9. DOIPubMedGoogle Scholar
- Lopez FA, Manglicmot JS, Schmidt TM, Yeh C, Smith HV, Relman DA. Molecular organization of Cyclospora-like organisms from baboons. J Infect Dis. 1999;179:670–6. DOIPubMedGoogle Scholar
- Sterling CR, Ortega YR. Cyclospora: an enigma worth unraveling. Emerg Infect Dis. 1999;5:48–53. DOIPubMedGoogle Scholar
- Carreno RA, Barta JR. An eimeriid origin of isosporoid coccidia with stieda bodies as shown by phylogenetic analysis of small subunit ribosomal RNA gene sequences. J Parasitol. 1999;85:77–83. DOIPubMedGoogle Scholar
- Eberhard ML, Pieniazek NJ, Arrowood MJ. Laboratory diagnosis of Cyclospora infections. Arch Pathol Lab Med. 1997;121:792–7.PubMedGoogle Scholar
- Visvesvara GS, Moura H, Kovacs-Nace E, Wallace S, Eberhard ML. Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. J Clin Microbiol. 1997;35:730–3.PubMedGoogle Scholar
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. DOIPubMedGoogle Scholar
- Strimmer K, von Haeseler A. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol Biol Evol. 1996;13:964–9.
- Swofford DL. PAUP*: Phylogenetic analysis using parsimony (and other methods). 1998. Ver 4.0b1. Sunderland (MA): Sinauer Associates.