Volume 8, Number 12—December 2002
Research
Meteorologic Influences on Plasmodium falciparum Malaria in the Highland Tea Estates of Kericho, Western Kenya
Abstract
Recent epidemics of Plasmodium falciparum malaria have been observed in high-altitude areas of East Africa. Increased malaria incidence in these areas of unstable malaria transmission has been attributed to a variety of changes including global warming. To determine whether the reemergence of malaria in western Kenya could be attributed to changes in meteorologic conditions, we tested for trends in a continuous 30-year monthly malaria incidence dataset (1966–1995) obtained from complete hospital registers at a Kenyan tea plantation. Contemporary monthly meteorologic data (1966–1995) that originated from the tea estate meteorologic station and from global climatology records were also tested for trends. We found that total hospital admissions (malaria and nonmalaria) remained unchanged while malaria admissions increased significantly during the period. We also found that all meteorologic variables showed no trends for significance, even when combined into a monthly suitability index for malaria transmission. We conclude that climate changes have not caused the highland malaria resurgence in western Kenya.
Highland malaria has returned to the tea estates of western Kenya after an absence of nearly 30 years (1–3). Altitude and weather influence malaria epidemiology in highland areas because of the slowing of parasite development within the anopheline vectors at lower temperatures (4). Increased malaria incidence in unstable transmission areas has been variously attributed to changes in land-use patterns (5); population migration (6,7); changes in mosquito vector populations (8); breakdown in provision of health services (9), especially insecticide spraying (10,11); drug resistance (12–16); and meteorologic changes (17,18), particularly global warming (19–25).
We investigated whether climate changes could be implicated in the reemergence of malaria in a unique 30-year malaria and meteorologic time series, collected from the health-care system on a tea plantation in the western highlands of Kenya. Our detailed substudy included site-specific meteorologic and malariometric data from a larger analysis of trends in meteorologic conditions across East Africa from 1911 to 1995 (26–28). Our previous studies have also examined various aspects of the epidemiology of malaria in the Kenyan highlands (29,30).
Study Site and Clinical Data
Long-term malaria illness and total hospital admissions data (January 1966–December 1995) exist from a large tea plantation in Kericho, Kenya, which is operated by Brooke Bond Kenya Ltd. (3,31,32). The plantation, located in the western Rift Valley highlands, covers an area of approximately 141 km2 and ranges from 1,780 to 2,225 m above mean sea level. Epidemic malaria was first recorded on the Kericho tea estates during World War II and was eventually controlled by a combination of mass administration of proguanil and residual insecticide spraying during the late 1940s (2). Currently, the Brooke Bond Kenya Ltd. plantation consists of approximately 20 separate tea estates with a total of 50,000 employees and dependents, who receive their medical care from the company-operated health systems. The company hospital maintains a 24-hour, 7-day clinical admission service for patients who need intensive clinical management. Stained blood smears from patients with suspected malaria are examined; this procedure, in combination with further supportive clinical and laboratory procedures, is used to confirm a primary malaria diagnosis. Case numbers in Kericho can be treated as incidence figures since the population eligible for health care remained at approximately 50,000 during the recording period (32). No centralized preventive chemoprophylaxis, vector control, or bed-net distribution has been implemented since the late 1950s. A substantial minority of the tea estate workers originate from the holoendemic Lake Victoria area and travel back and forth intermittently to their home areas; however, this travel pattern has been occurring since the road was surfaced in the 1950s and has not changed recently. This study was conducted under a protocol approved by the Kenyan National Ethical Review Committee (SSC 484) and the U.S. Army Office of the Surgeon General (WRAIR 682).
Two meteorologic datasets were compiled. Point locality measurements of mean monthly temperature (°C) and monthly total rainfall (mm) were obtained from the Tea Research Foundation meteorologic station on the Kericho tea estates for the 1966–1995 period. Climate data were also obtained from a global 0.5 x 0.5° (approximately 55 x 55 km [3,025 km2] at the equator) gridded dataset of monthly terrestrial surface climate for the 1966–1995 period (33,34) (available from: URL: http://www.cru.uea.ac.uk/link). The dataset was used to ensure that results from the single meteorologic station were in agreement with data from a wider geographic area; this procedure also allowed a wider range of climate variables, including temperature extremes, to be tested. Primary variables of precipitation (mm), mean temperature (°C), and diurnal temperature range (°C) were available and interpolated from extensive meteorologic station data by using angular distance-weighted averaging of anomaly fields. The secondary variable of vapor pressure was also provided, interpolated where available, and calculated from primary variables, when the coverage of meteorologic stations was insufficient. Minimum and maximum monthly temperature estimates were created by subtracting or adding, respectively, half the diurnal temperature range from mean monthly temperature. Time series were derived by using an extraction routine developed in ENVI (Research Systems Inc., Boulder, CO) with georeferencing information for Kericho (0.33°S, 35.37°E), obtained from Encarta (Microsoft, Seattle, WA).
To investigate whether a combination of meteorologic conditions was changing and thus facilitating the resurgence of malaria, we also categorized months as suitable for Plasmodium falciparum transmission if they had a mean monthly temperature exceeding 15°C (since temperatures experienced by the indoor resting Anopheles gambiae vectors are likely to be 3°C–5°C higher) and monthly rainfall totals exceeding 152 mm (1,4) by using the gridded climatology data. The numbers of suitable months for transmission were summed, totaled for each year, and tested for the 1966–1995 period.
Statistical Analyses
To test for trends in the climate and malaria suitability time series, we estimated the following regression equation:
If the time series y can be characterized as the sum of a stationary stochastic process and a linear time trend, then the appropriate test for the trend is a t test on β in (1). If the series is a random walk, however, or a more complex stochastically trending process, the critical levels for the distribution of the t score in this regression are much greater than usual (35), and alternative tests should be employed. Since many climate time series contain a stochastically trending component (36), the nature of the series must be explored before testing for climate change. This methodologic issue complicates the evaluation of the significance of trends established with standard regression procedures often used in such studies.
If γ =0 (a unit root in the autoregressive process) and β =0, then y is a random walk. The random walk may also have a deterministic drift term (α≠0). In either case, however, the series is nonstationary, and classical regression inference does not apply. The nonstandard distributions of α, β, and γ have been tabulated by Dickey and Fuller (37,38). We first tested for the presence of a unit root by evaluating the t statistic for γ against its nonstandard distribution. The critical value for this so-called Augmented Dickey-Fuller at the 5% level is -3.45. Values of the t statistic for γ more negative than this critical value indicate that the series is not a random walk and vice versa. If the null hypothesis is rejected, then the t statistics associated with α and β are normally distributed. If the unit root hypothesis is accepted, then these statistics also have nonstandard distributions. The correct test for a trend is then the t test on α in (1) with the omission of the linear trend. The test’s critical value at the 5% significance level is 2.54. The results of these tests are presented in the Table.
We also regressed temperature and rainfall data from the meteorologic station at Kericho on the same variables from the interpolated climatology (33,34) by using a variety of formulations including levels, logarithms, and a regression adjusted for heteroscedasticity. We then tested whether the slope coefficients were significantly different from unity, which should not be the case if the gridded dataset is a good proxy for the climate at Kericho.
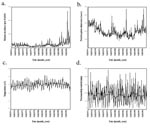
During the period 1966–1995, malaria incidence increased significantly (p=0.0133) while total (i.e., malarial and other) admissions to the tea estate hospital showed no significant change (Table and Figure 1a,b). Measurements of mean monthly temperature and total monthly rainfall also showed no significant changes (Table and Figure 1c,d). Similar results were shown by the climatology data interpolated from a wider area. Mean, maximum, and minimum monthly temperatures; precipitation; and vapor pressure all demonstrated no significant trends (Table; Figure 2a,b,c). Moreover, the interpolated climatology data, when transformed into month of malaria transmission suitability (1,4), again showed no significant changes (Table; and Figure 2d).
Results were very similar, though significance levels varied, between the three formulations of the regression model that compared the local meteorologic station data and those from the interpolated climatology data (33,34). The coefficient for the regression of the meteorologic station rainfall data on the interpolated climatology precipitation data is in every case not significantly different from unity. Significance levels are 10% for the model in levels, 18% for the heteroscedasticity-adjusted model, and 96% for the logarithmic model. In the regression of the two temperature series, however, the coefficient is significantly different from unity in every case, as is a joint test statistic for the two slope coefficients.
The resurgence of P. falciparum malaria in the East African highlands (3,8,18,26, 40–44) has led several researchers to speculate that climate change is a predominant cause (23,45–50). On the basis of these studies, which have been disputed by experts in vector-borne disease biology (10,27–29, 51,52), and some biological modeling, which has been robustly criticized (53), the International Panel on Climate Change has recently concluded with “medium-to-high confidence” that there will be a net increase in the range and incidence of malaria (49); the results of our work do not support these conclusions.
Malaria incidence increased significantly (p=0.0133) during the 1966–1995 period, while total admissions remained unchanged. Besides an increase in local malaria transmission, two other factors may have influenced the increase in malaria hospitalizations. An increase in malaria severity indicated by an increased case-fatality rate (from 1.3% in the 1960s to 6% in the 1990s) is most likely linked to chloroquine resistance, which we believe to be the probable cause of much of the overall increase in malaria transmission (32). Travel to and from the Lake Victoria region by a minority of the tea estate workers also exerts an upward influence on malaria transmission in Kericho since such travel increases the numbers of workers asymptomatically carrying gametocytes, which infect mosquitoes for further human infection. This complex topic is the subject of a future publication.
All climate variables, whether from the Kericho tea estate meteorologic station or the pixel covering Kericho in the global climatology dataset showed no significant trends, despite the fact that equivalence tests showed some significant differences between the temperature time series—findings that are in agreement with a broader geographic analysis of East African data from 1911 to 1995 (26) and lend support to the appropriateness of interpolated climate data for use in these investigations. We also think that, when examining trends in meteorologic phenomena, epidemiologists should use more robust statistical techniques for the reasons outlined in the methods. The results of this detailed examination of coincident empirical data do not support the widespread, recent speculation regarding malaria resurgences in response to climate change. No aspect of climate has changed significantly—neither the temperature extremes (maximum and minimum) nor the periods when meteorologic data were transformed into months when malaria transmission is possible. Further study has also shown that variability in these meteorologic variables, independent of any longer term trends, has decreased (54). We must therefore look elsewhere for the causes of these resurgences (27,28,32). These factors are likely to vary. In Kericho, however, increased chloroquine resistance has been strongly argued to be the cause, since all other relevant environmental and sociologic factors are unchanged (32).
The attraction of the global warming hypothesis as an explanation of highland malaria is the existence of a continental trend toward global warming coincident with a trend toward increasing malaria incidence in several parts of Africa, ranging from Senegal (13,14) to Madagascar (10). Where such malaria increases have been examined in detail, however, alternative explanations such as discontinuation of anti-vector measures in Madagascar (10) or chloroquine resistance in Senegal appear to be more likely causes (13,14). Malaria epidemiology is greatly influenced by a range of local factors, making a consistent continent-wide explanation seem unlikely (28,52).
We do not argue that meteorologic conditions have no immediate impact on the seasonal dynamics and incidence of malaria or that climate change is probably not an important future concern in public health. Rather we urge some caution in the interpretation of synonymous changes in climate over wider areas and local changes in malaria incidence.
Col. G. Dennis Shanks is the former director of the U.S. Army Component of the Armed Forces Institute of Medical Research in Bangkok, Thailand, which is a part of the Walter Reed Army Institute of Research. He is a physician trained in pediatrics and tropical medicine whose main professional interests are malaria chemotherapy, malaria epidemiology, and clinical trials in developing countries.
Acknowledgments
The authors acknowledge the management and staff of Brooke Bond Kenya Ltd. and its Central Hospital in Kericho, whose outstanding medical system made this study possible, and the support of the Kenya Medical Research Institute in Nairobi, Kenya. We also thank Wilson K. Ngetich for supplying the local meteorologic data.
SIH is currently supported as an advanced training fellow by the Welcome Trust (#056642). RWS is a senior Wellcome Trust fellow (#033340).
References
- Garnham PCC. Malaria epidemics at exceptionally high altitudes in Kenya. BMJ. 1945;11:45–7. DOIGoogle Scholar
- Strangeways-Dixon D. Paludrine (proguanil) as a malarial prophylactic amongst African labour in Kenya. East Afr Med J. 1950;27:127–30.PubMedGoogle Scholar
- Malakooti MA, Biomndo K, Shanks GD. Reemergence of epidemic malaria in the highlands of western Kenya. Emerg Infect Dis. 1998;4:671–6.PubMedGoogle Scholar
- Garnham PCC. The incidence of malaria at high altitudes. J Natl Malar Soc. 1948;7:275–84.PubMedGoogle Scholar
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Trop Med Int Health. 2000;5:263–74. DOIPubMedGoogle Scholar
- Van der Stuyft P, Manirankunda L, Delacollette C. L'approche de risque dans le diagnostic du paludisme-maladie en regions d'altitude. Ann Soc Belg Med Trop. 1993;73:81–9.PubMedGoogle Scholar
- Bashford G, Richens J. Travel to the coast by highlanders and its implications for malaria control. P N G Med J. 1992;35:306–7.PubMedGoogle Scholar
- Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Highland malaria in Uganda: prospective analysis of an epidemic associated with El Niño. Trans R Soc Trop Med Hyg. 1999;93:480–7. DOIPubMedGoogle Scholar
- Pitt S, Pearcy BE, Stevens RH, Sharipov A, Satarov K, Banatvala N. War in Tajikistan and re-emergence of Plasmodium falciparum. Lancet. 1998;352:1279. DOIPubMedGoogle Scholar
- Mouchet J, Manguin S, Sircoulon J, Laventure S, Faye O, Onapa AW, Evolution of malaria in Africa for the past 40 years: impact of climatic and human factors. J Am Mosq Control Assoc. 1998;14:121–30.PubMedGoogle Scholar
- Mouchet J. L'origine des épidémies de paludisme sur les Plateaux de Madagascar et les montagnes d'Afrique de L'est et du Sud. Bull Soc Pathol Exot. 1998;91:64–6.PubMedGoogle Scholar
- Warsame M, Wernsdorfer WH, Huldt G, Björkman A. An epidemic of Plasmodium falciparum malaria in Balcad, Somalia, and its causation. Trans R Soc Trop Med Hyg. 1995;89:142–5. DOIPubMedGoogle Scholar
- Trape JF. Impact of chloroquine resistance on malaria mortality. Comptes Rendus de l'Academie des Sciences, Paris. 1998;321:689–97.
- Trape JF. The public health impact of chloroquine resistance in Africa. Am J Trop Med Hyg. 2001;64:12–7.PubMedGoogle Scholar
- Bødker R, Kisinza W, Malima R, Msangeni H, Lindsay S. Resurgence of malaria in the Usambara mountains, Tanzania, an epidemic of drug-resistant parasites. Glob Change Hum Health. 2000;1:134–53. DOIGoogle Scholar
- Etchegorry MG, Matthys F, Galinski M, White NJ, Nosten F. Malaria epidemic in Burundi. Lancet. 2001;357:1046–7. DOIPubMedGoogle Scholar
- Brown V, Issak MA, Rossi M, Barboza P, Paugam A. Epidemic of malaria in north-eastern Kenya. Lancet. 1998;352:1356–7. DOIPubMedGoogle Scholar
- van der Hoek W, Konradsen F, Perera D, Amerasinghe PH, Amerasinghe FP. Correlation between rainfall and malaria in the dry zone of Sri Lanka. Ann Trop Med Parasitol. 1997;91:945–9. DOIPubMedGoogle Scholar
- Loevinsohn ME. Climatic warming and increased malaria incidence in Rwanda. Lancet. 1994;343:714–8. DOIPubMedGoogle Scholar
- Bouma MJ, Dye C, Van der Kaay HJ. Falciparum malaria and climate change in the northwest Frontier province of Pakistan. Am J Trop Med Hyg. 1996;55:131–7.PubMedGoogle Scholar
- Lindsay SW, Birley MH. Climate change and malaria transmission. Ann Trop Med Parasitol. 1996;90:573–88.PubMedGoogle Scholar
- Lindsay SW, Martens WJM. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76:33–45.PubMedGoogle Scholar
- McMichael AJ, Haines A, Sloof R, Kovats S. Climate change and human health. Geneva:World Health Organization; 1996.
- Martens P, Kovats RS, Nijhof S, de Vries P, Livermore MTJ, Bradley DJ, Climate change and future populations at risk of malaria. Glob Environ Change. 1999;9:89–107. DOIGoogle Scholar
- National Research Council. Under the weather: climate, ecosystems, and infectious disease. Washington: The Council; 2001.
- Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, Climate change and the resurgence of malaria in the East African highlands. Nature. 2002;415:905–9. DOIPubMedGoogle Scholar
- 2Hay SI, Cox J, Rogers DJ, Randolph SE, Stern DI, Shanks GD, et al. East African highland malaria resurgence independent of climate change. Directions in Science 2002;1:82–5.
- Hay SI, Rogers DJ, Randolph SE, Stern DI, Cox J, Shanks GD, Hot topic or hot air? Climate change and malaria resurgence in African highlands. Trends Parasitol. 2002;18. In press.
- Hay SI, Noor AM, Simba M, Busolo M, Guyatt HL, Ochola SA, The clinical epidemiology of malaria in the highlands of Western Kenya. Emerg Infect Dis. 2002;8:543–8.PubMedGoogle Scholar
- Hay SI, Simba M, Busolo M, Noor AM, Guyatt HL, Ochola SA, Defining and detecting malaria epidemics in the highlands of western Kenya. Emerg Infect Dis. 2002;8:555–62.PubMedGoogle Scholar
- 3Hay SI, Myers MF, Burke DS, Vaughn DW, Endy T, Ananda N, et al. Etiology of interepidemic periods of mosquito-borne disease. Proc Natl Acad Sci U S A 2000;97:9335–9.
- Shanks GD, Biomndo K, Hay SI, Snow RW. Changing patterns of clinical malaria since 1965 among a tea estate population located in the Kenyan highlands. Trans R Soc Trop Med Hyg. 2000;94:253–5. DOIPubMedGoogle Scholar
- New M, Hulme M, Jones P. Representing twentieth-century space-time climate variability. Part I: development of a 1961-90 mean monthly terrestrial climatology. J Climatol. 1999;12:829–57. DOIGoogle Scholar
- New M, Hulme M, Jones P. Representing twentieth-century space-time climate variability. Part II: development of 1901-1996 monthly grids of terrestrial surface climate. J Climatol. 2000;13:2217–38. DOIGoogle Scholar
- Granger CWJ, Newbold P. Spurious regressions in econometrics. J Econom. 1974;2:111–20. DOIGoogle Scholar
- Stern DI, Kaufmann RK. Detecting a global warming signal in hemispheric temperature series: a structural time series analysis. Clim Change. 2000;47:411–38. DOIGoogle Scholar
- Dickey DA, Fuller WA. Distribution of the estimators for autoregressive time series with a unit root. J Am Stat Assoc. 1979;74:427–31. DOIGoogle Scholar
- Dickey DA, Fuller WA. Likelihood ratio statistics for autoregressive processes. Econometrica. 1981;49:1057–72. DOIGoogle Scholar
- Box G, Pierce D. Distribution of autocorrelations in autoregressive moving average time series models. J Am Stat Assoc. 1970;65:1509–26. DOIGoogle Scholar
- Matola YG, White GB, Magayuka SA. The changed pattern of malaria endemicity and transmission at Amani in the eastern Usambara Mountains, north-eastern Tanzania. J Trop Med Hyg. 1987;90:127–34.PubMedGoogle Scholar
- Marimbu J, Ndayiragije A, Le Bras M, Chaperon J. Environment and malaria in Burundi: apropos of a malaria epidemic in a non-endemic mountainous region. Bull Soc Pathol Exot. 1993;86:399–401.PubMedGoogle Scholar
- Some E. Effects and control of highland malaria epidemic in Uasin Gishu District, Kenya. East Afr Med J. 1994;71:2–8.PubMedGoogle Scholar
- Tulu AN. Determinants of malaria transmission in the highlands of Ethiopia: the impact of global warming on mortality and morbidity ascribed to malaria. In: London School of Hygiene and Tropical Medicine. London:University of London; 1996.
- Kilian AHD, Langi P, Talisuna A, Kabagambe G. Rainfall pattern, El Niño and malaria in Uganda. Trans R Soc Trop Med Hyg. 1999;93:22–3. DOIPubMedGoogle Scholar
- Epstein PR, Diaz HF, Elias S, Grabherr G, Graham NE, Martens WJM, Biological and physical signs of climate change: focus on mosquito-borne diseases. Bull Am Meteorol Soc. 1998;79:409–17. DOIGoogle Scholar
- Martens P. How will climate change affect human health? Am Sci. 1999;87:534–41.
- Patz JA, Lindsay SW. New challenges, new tools: the impact of climate change on infectious diseases. Curr Opin Microbiol. 1999;2:445–51. DOIPubMedGoogle Scholar
- Bonora S, De Rosa FG, Boffito M, Di Perri G, Rossati A. Rising temperature and the malaria epidemic in Burundi. Trends Parasitol. 2001;17:572–3. DOIPubMedGoogle Scholar
- McCarthy JJ, Canziani OF, Leary NA, Dokken DJ, White KS. Climate change 2001: impacts, adaptation, and vulnerability—contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge Univ. Press; 2001.
- Patz JA, Reisen WK. Immunology, climate change and vector-borne diseases. Trends Immunol. 2001;22:171–2. DOIPubMedGoogle Scholar
- Reiter P. Global-warming and vector-borne disease in temperate regions and at high altitude. Lancet. 1998;351:839. DOIPubMedGoogle Scholar
- Reiter P. Climate change and mosquito-borne disease. Environ Health Perspect. 2001;109:141–61. DOIPubMedGoogle Scholar
- Rogers DJ, Randolph SE. The global spread of malaria in a future, warmer world. Science. 2000;289:1763–6. DOIPubMedGoogle Scholar
- Rogers DJ, Randolph SE, Snow RW, Hay SI. Satellite imagery in the study and forecast of malaria. Nature. 2002;415:710–5. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 8, Number 12—December 2002
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
G. Dennis Shanks, Armed Forces Institute of the Medical Sciences, APO AP 96546 USA; fax: 66 2 2476030; ; or Simon I. Hay, TALA Research Group, Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, U.K.; fax: 44 1865 271243;
Top