Volume 14, Number 5—May 2008
Dispatch
Serologic Evidence for Novel Poxvirus in Endangered Red Colobus Monkeys, Western Uganda
Figure 1
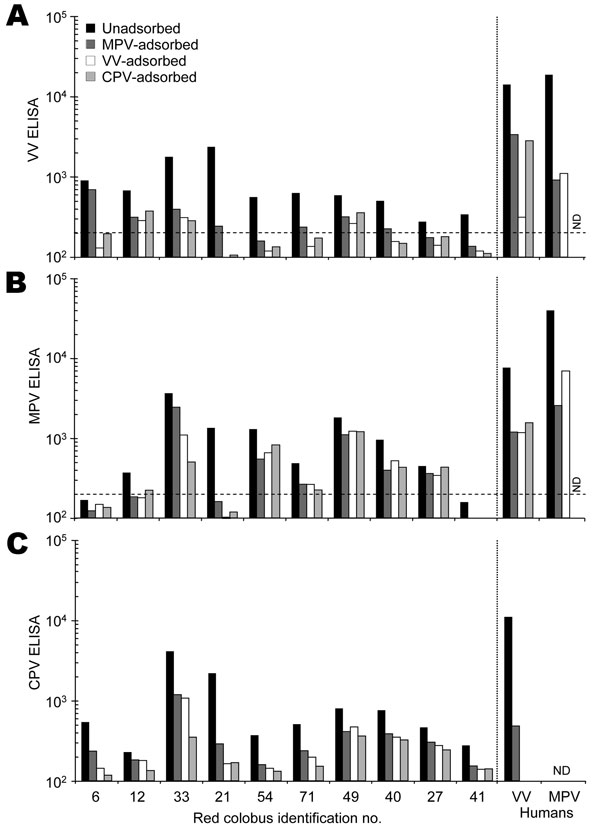
Figure 1. Serologic characterization of red colobus to Orthopoxvirus antigens. Plasma samples were collected from 31 red colobus, and 10 samples with detectable antibody responses to vaccinia virus (VV) antigens (Appendix Figure) were chosen for further analysis. Plasma samples were tested for specificity by a postadsorption ELISA (7) in which samples were either unadsorbed or preadsorbed with monkeypox virus (MPV), vaccinia virus (VV), or cowpox virus (CPV) antigens prior to performing an ELISA on A) VV-, B) MPV-, or C) CPV-coated ELISA plates. The results obtained by using plasma from a VV-immune human study participant (VV human) and a MPV-immune participant (MPV human) are shown for comparison. The dashed line indicates the cut-off value for a seropositive antibody response (200 ELISA units). ND, not determined.
References
- Regnery RL. Poxviruses and the passive quest for novel hosts. Curr Top Microbiol Immunol. 2007;315:345–61.PubMedGoogle Scholar
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. DOIPubMedGoogle Scholar
- Reed KD, Melski JW, Graham MB, Regnery RL, Sotir MJ, Wegner MV, The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–50. DOIPubMedGoogle Scholar
- Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–11.PubMedGoogle Scholar
- Struhsaker TT. Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville (FL): University Press of Florida; 1997.
- Chapman CA, Struhsaker TT, Lambert JE. Thirty years of research in Kibale National Park, Uganda, reveals a complex picture for conservation. Int J Primatol. 2005;26:539–55. DOIGoogle Scholar
- Dubois MD, Slifka MK. Retrospective analysis of monkeypox infection. Emerg Infect Dis. 2008 Apr; [Epub ahead of print].
- Downie AW, Taylor-Robinson CH, Caunt AE, Nelson GS, Manson-Bahr PEC, Matthews THC. Tanapox: a new disease caused by a poxvirus. BMJ. 1971;1:363–8.PubMedGoogle Scholar
- Jezek Z, Arita I, Szceniowski M, Paluku KM, Kalisa R, Nakano JH. Human tanapox in Zaire: clinical and epidemiological observations on cases confirmed by laboratory studies. Bull World Health Organ. 1985;63:1027–35.PubMedGoogle Scholar
- Struhsaker TT. Vocalizations, phylogeny and palaeogeography of red colobus monkeys (Colobus badius). Afr J Ecol. 1981;19:265–83. DOIGoogle Scholar
- Ting N. Mitochondrial relationships and divergence dates of the African colobines: evidence of miocene origins for the living colobus monkeys. J Hum Evol. In press.
- Struhsaker TT. Variation in adult sex ratios of red colobus monkey social groups: implications for interspecific comparisons. In: Kappeler PM, editor. Primate males: causes and consequences of variation in group composition. Cambridge (UK): Cambridge University Press; 2000. p. 108–19.
- Goldberg TL, Gillespie TR, Rwego IB, Kaganzi C. Killing of a pearl-spotted owlet (Glaucidium perlatum) by male red colobus monkeys (Procolobus tephrosceles) in a forest fragment near Kibale National Park, Uganda. Am J Primatol. 2006;68:1007–11. DOIPubMedGoogle Scholar
- Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDS epidemic update. Geneva: The Programme; 2007.