Volume 21, Number 10—October 2015
Dispatch
Spatiotemporal Patterns of Schistosomiasis-Related Deaths, Brazil, 2000–2011
Abstract
We analyzed spatiotemporal patterns of 8,756 schistosomiasis-related deaths in Brazil during 2000–2011 and identified high-risk clusters of deaths, mainly in highly schistosomiasis-endemic areas along the coast of Brazil’s Northeast Region. Schistosomiasis remains a neglected public health problem with a high number of deaths in disease-endemic and emerging focal areas.
Schistosomiasis is a neglected tropical disease (NTD) caused by infection with Schistosoma spp. trematodes and a public health problem worldwide, mainly in areas without access to safe drinking water and adequate sanitation (1,2). Brazil is the most heavily affected country in the Americas (1), with about 2.5 million–6 million infected persons (3) and 700–800 deaths are reported annually (4). The disease’s continued expansion because of human migration from schistosomiasis-endemic to -nonendemic areas means schistosomiasis is increasingly considered an emerging disease in Brazil (5). Using different spatial analytical approaches, we examined spatiotemporal patterns and determined high-risk clusters for schistosomiasis-related deaths in Brazil.
We analyzed death certificate data obtained from the Brazilian Mortality Information System (http://tabnet.datasus.gov.br/cgi/sim/dados/cid10_indice.htm) and used the 5,565 municipalities of residence in Brazil as geographic units of analysis. We included deaths occurring during 2000–2011 for which schistosomiasis (code B65, International Classification of Diseases, Tenth Revision [ICD-10]) was recorded as underlying or associated (contributing) causes of death (multiple causes of death) (6). Deaths with unknown municipality of residence were excluded. Population data at the municipality level were obtained from the Brazilian Institute of Geography and Statistics (http://tabnet.datasus.gov.br/cgi/deftohtm.exe?ibge/cnv/popuf.def).
To minimize random variations, especially in municipalities with small populations and rare events, we calculated average annual death rates (per 100,000 inhabitants) at the municipality level over the entire period (average annual number of deaths/population size during the middle of the study period). We then calculated smoothed death rates by using the local empirical Bayes method (Technical Appendix). Presence of global and local spatial autocorrelation was evaluated by using Global Moran’s I and Local Moran’s I statistics (7), respectively (Technical Appendix). A retrospective space-time scan statistic (8) was used to identify statistically significant high-risk spatiotemporal clusters (Technical Appendix). Primary (i.e., most likely) and secondary clusters were detected by using the log-likelihood ratio test; clusters with maximum log-likelihood ratios were considered primary.
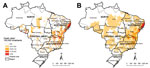
A total of 12,491,280 deaths were recorded in Brazil for 2000–2011. Schistosomiasis was identified in 8,756 deaths (0.07%), as an underlying cause in 6,319 (72.2%) and as an associated cause in 2,437 (27.8%) deaths. The nationwide average annual crude rate of death atttibuted to schistosomiasis (for underlying and associated causes) was 0.39 deaths (95% CI 0.37–0.42) per 100,000 inhabitants. Of 5,565 municipalities, ≈1,225 (≈22%) recorded >1 schistosomiasis-related death. Spatial distribution of average annual crude and smoothed death rates at the municipal level showed a concentration of municipalities with higher death rates (>1.0 death/100,000 inhabitants) along the east coast of Brazil’s Northeast Region, extending to the states of Minas Gerais and Espírito Santo (Figure 1, panels A, B).
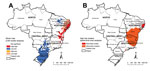
Global Moran’s I index showed significant positive spatial autocorrelation (0.32, p<0.01). Local Moran’s I identified high-risk clusters (classified as “High/High”) of schistosomiasis-related deaths, corresponding mainly to municipalities with high rates shown in the descriptive maps (Figure 2, panel A). As with the concentration of high death rates, major high-risk clusters included a large geographic area on the east coast of the Northeast region (Figure 2, panel A).
Scan space-time analysis identified 3 spatiotemporal high-risk clusters (Figure 2, panel B; Table). Primary clusters were detected during 2001–2006 and represented 2,150 deaths in 191 municipalities distributed in 3 states in the Northeast region. The relative risk was 12.96 (p<0.01), and the annual crude rate was 4.0 deaths/100,000 inhabitants. Secondary clusters were located in the Southeast and Northeast regions (Figure 2, panel B; Table).
In this nationwide population-based study in Brazil, we found a heterogeneous geographic pattern of schistosomiasis-related deaths. Independently from the spatial statistical approach, high-risk clusters for schistosomiasis-related deaths were identified mainly in the highly schistosomiasis-endemic areas along the east coast of the Northeast Region, particularly in the states of Alagoas, Pernambuco, Sergipe, and Bahia and extending north of Minas Gerais and Espírito Santo States in the Southeast (4,9,10). These areas have ecologic and geographic conditions favorable to schistosomiasis: presence and proliferation of the intermediate snail host, poor living conditions, and inadequate sanitation (10). Reducing severe forms of schistosomiasis will require controlling transmission by implementing measures such as promoting basic sanitation and health education (4,11).
We also identified high rates of schistosomiasis-related deaths in areas where the disease is not endemic and has no focal transmission. The continuing emergence of schistosomiasis, characterized by the appearance of new foci in nonendemic areas and by urbanization of the disease, may be related to internal migration, increasing urban agglomeration, wide distribution of intermediate hosts, and discontinuation of disease control measures (9). High levels of internal migratory movement, the spread of snail intermediate hosts, and poor sanitary conditions increase the risk for establishing new foci in Brazil (9,12). For example, Rondônia state in North Brazil recorded increasing numbers of confirmed cases in recent years (13). Most cases and deaths in this state were not autochthonous but were identified in migrants coming from schistosomiasis-endemic regions of Brazil (13). The presence of potential intermediate hosts has been confirmed in Rondônia, increasing the possibility that the disease will establish there (12,13). In other regions of the world, transmission seems to establish in non–disease-endemic areas; on the island of Corsica (France), several tourists have been infected with Schistosoma haematobium while bathing in local rivers (14).
Although schistosomiasis is a disease typical of poor rural areas, intensified urbanization in recent decades has led to increasing numbers of urban cases and deaths (11,15). Municipalities that recorded the highest number of deaths were concentrated in Brazilian state capitals, especially in São Paulo (São Paulo State), Recife (Pernambuco State), Maceió (Alagoas State), and Belo Horizonte (Minas Gerais State). Most cases probably originated with persons coming from schistosomiasis-endemic rural areas and migrating to capital cities and metropolitan regions in search of improved living conditions and increased access to specialized health services (11).
Furthermore, development and management of water resources projects can introduce schistosomiasis into areas not previously endemic for the disease (2). The transposition of the largest river in the Northeast Region (São Francisco River), set to begin in 2016, may contribute to disease outbreaks through dispersion of intermediate hosts to areas not previously schistosomiasis endemic and through increased migratory activities of construction workers and their families (4).
Our study is subject to limitations. Because we used secondary death data, deaths may be underreported (4), despite progress achieved in registration of deaths (estimated proportion of deaths reported increased from 91.0% in 2000 to 94.2% in 2011; http://tabnet.datasus.gov.br/cgi/idb2012/a1801b.htm). Furthermore, schistosomiasis as an underlying cause of death may be underreported because it could be coded as a complication or illness associated with schistosomiasis (e.g., gastrointestinal bleeding, portal hypertension, esophageal varices) (4,11). To reduce this error, we collected information from data showing multiple causes of death (underlying and associated causes) and identified all death certificates that mentioned schistosomiasis. In addition, identifying areas of high transmission of disease by using death data must be approached with care. Schistosomiasis is a chronic disease, and death may result from an infection acquired many years earlier (4). Because of geographic migration of infected persons, place of residence at time of death may not be the place where the infection was acquired (5). Another limitation is the uncertainty of population estimates during intercensus years used in calculations of rates, especially estimates for years far from census years (2000 and 2010).
Our results indicate spatiotemporal heterogeneity of schistosomiasis-related deaths in Brazil over a 12-year period. High-risk clusters were located mainly in highly schistosomiasis-endemic areas. Disease control programs should increase geographic coverage, intensify and focus efforts to reduce transmission and prevent severe illnesses and deaths, and prevent establishment of schistosomiasis in areas where it is not yet endemic.
Mr. Martins-Melo is a doctoral student in the Department of Community Health, School of Medicine, Federal University of Ceará, Brazil. His research interests include the epidemiology of neglected and poverty-related diseases.
Acknowledgment
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) for granting a PhD scholarship to F.R.M. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil) for a research fellowship to J.H.
References
- World Health Organization. Schistosomiasis. Geneva: The Organization. 2014 [cited 2014 Jul 05]. http://www.who.int/mediacentre/factsheets/fs115/en/
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–25. DOIPubMedGoogle Scholar
- Katz N, Peixoto SV. Critical analysis of the estimated number of schistosomiasis mansoni carriers in Brazil [in Portuguese]. Rev Soc Bras Med Trop. 2000;33:303–8. DOIPubMedGoogle Scholar
- Martins-Melo FR, Pinheiro MC, Ramos AN Jr, Alencar CH, Bezerra FS, Heukelbach J. Trends in schistosomiasis-related mortality in Brazil, 2000–2011. Int J Parasitol. 2014;44:1055–62. DOIPubMedGoogle Scholar
- Kloos H, Correa-Oliveira R, dos Reis DC, Rodrigues EW, Monteiro LA, Gazzinelli A. The role of population movement in the epidemiology and control of schistosomiasis in Brazil: a preliminary typology of population movement. Mem Inst Oswaldo Cruz. 2010;105:578–86. DOIPubMedGoogle Scholar
- Redelings MD, Sorvillo F, Simon P. A comparison of underlying cause and multiple causes of death: US vital statistics, 2000–2001. Epidemiology. 2006;17:100–3. DOIPubMedGoogle Scholar
- Anselin L. Local indicators of spatial association–LISA. Geogr Anal. 1995;27:93–115. DOIGoogle Scholar
- Amaral RS, Tauil PL, Lima DD, Engels D. An analysis of the impact of the Schistosomiasis Control Programme in Brazil. Mem Inst Oswaldo Cruz. 2006;101(Suppl 1):79–85. DOIPubMedGoogle Scholar
- Resendes AP, Souza-Santos R, Barbosa CS. Hospitalization and mortality from mansoni schistosomiasis in the state of Pernambuco, Brazil, 1992/2000 [in Portuguese]. Cad Saude Publica. 2005;21:1392–401. DOIPubMedGoogle Scholar
- Nascimento GL, de Oliveira MR. Severe forms of schistosomiasis 402 mansoni: epidemiologic and economic impact in Brazil, 2010. Trans R Soc Trop Med Hyg. 2014;108:29–36. DOIPubMedGoogle Scholar
- Coura JR, Amaral RS. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Mem Inst Oswaldo Cruz. 2004;99(Suppl 1):13–9. DOIPubMedGoogle Scholar
- Normandes APF, Zan RA, Meneguetti DUO. Overview epidemiological of schistosomiasis in Rondônia State, Western Amazon, of 2001 to 2006 [in Portuguese]. R Epidemiol Control Infect. 2013;3:110.
- Holtfreter MC, Moné H, Müller-Stöver I, Mouahid G, Richter J. Schistosoma haematobium infections acquired in Corsica, France, August 2013. Euro Surveill. 2014;19:20821.PubMedGoogle Scholar
- Anaruma Filho F, Sant'Ana JM, dos Santos RF, Castagna CL. Environmental inducers of Schistosomiasis mansoni in Campinas, Brazil. Geospat Health. 2010;5:79–91. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 21, Number 10—October 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jorg Heukelbach, Department of Community Health, School of Medicine, Federal University of Ceará, 1608 Rua Prof Costa Mendes, 5th Fl, Fortaleza 60.430-140, Ceará, Brazil
Top