Volume 25, Number 10—October 2019
Research
Early Diagnosis of Tularemia by Flow Cytometry, Czech Republic, 2003–20151
Figure 1
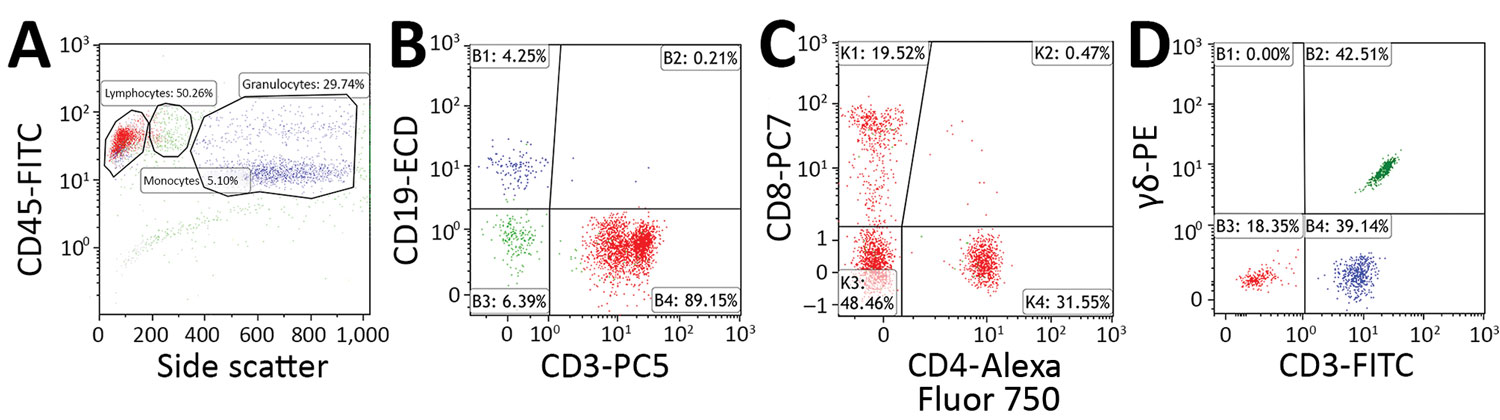
Figure 1. Flow cytometry gating strategy used to determine percentage of CD3+ lymphocytes that are CD3+/CD4–/CD8– T cells and γδ T cells in peripheral blood samples acquired from patients with suspected tularemia, Czech Republic, 2003–2015. A–C) Staining with CYTO-STAT tetraCHROME CD45-FITC/CD56-RD1 (phycoerythrin)/CD19-ECD/CD3-PC5, anti–CD4-Alexa Fluor 750, and anti–CD8-PC7 (Beckman Coulter, https://www.beckmancoulter.com). A) CD45 versus side scatter plot. Percentages of lymphocytes (red), monocytes (green), and granulocytes (blue) are indicated. In total, 3,000 lymphocytes were selected for further analysis. B) B cells (blue) and T cells (red) plotted according to their CD19 and CD3 expression. Percentages of cells within each quadrant are indicated. T cells were selected for further analysis. C) Percentage of CD3+ T cells not displaying CD4 and CD8 (CD4–/CD8–) determined with CD4 versus CD8 plots. Percentages of cells within each quadrant are indicated. D) Staining with anti–CD3-FITC and anti–T-cell receptor PAN γδ-PE (Beckman Coulter). After a side scatter and forward scatter plot (not shown), the percentage of lymphocytes that were CD3+/γδ T cells (green) were determined with a CD3 versus T-cell receptor pan–γδ plot. Percentages of cells within each quadrant are indicated. Flow cytometry was performed in the Immunology Laboratory of České Budějovice Hospital (České Budějovice, Czech Republic). ECD, phycoerythrin-Texas Red-X; FITC, fluorescein isothiocyanate; PC, phycoerythrin cyanine; PE, phycoerythrin.
1Preliminary data from this study were presented at the European Congress of Clinical Microbiology and Infectious Diseases; April 9–12, 2016; Amsterdam, the Netherlands (abstract no. O367).