Volume 26, Number 9—September 2020
Research
Evaluation of World Health Organization–Recommended Hand Hygiene Formulations
Figure
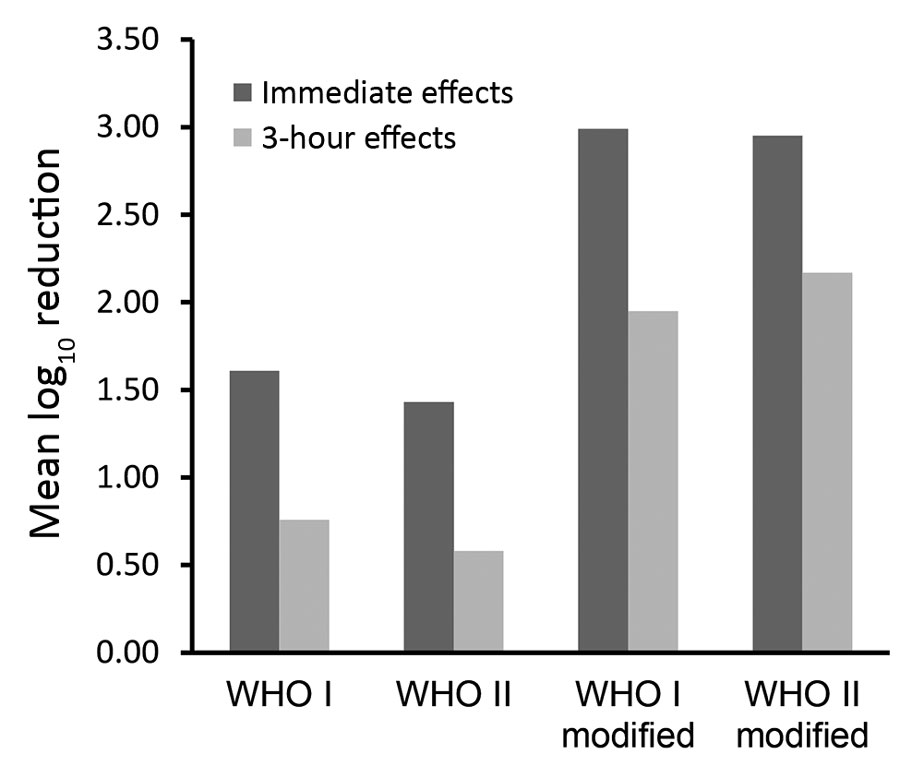
Figure. Comparison of the immediate and 3-hour effects of the WHO-recommended (WHO I and WHO II) and modified (WHO I modified and WHO II modified) formulations among 24 volunteers after a 3-minute surgical hand preparation according to European Norm 12791 (3). WHO I: ethanol 80% vol/vol + glycerol 1.45% vol/vol + hydrogen peroxide 0.125% vol/vol. WHO II: isopropanol 75% vol/vol + glycerol 1.45% vol/vol + hydrogen peroxide 0.125% vol/vol. WHO I modified: ethanol 80% wt/wt + glycerol 0.5% vol/vol + hydrogen peroxide 0.125% vol/vol. WHO II modified: isopropanol 75% wt/wt + glycerol 0.5% vol/vol + hydrogen peroxide 0.125% vol/vol. WHO, World Health Organization.
References
- World Health Organization. WHO guidelines on hand hygiene in health care. Geneva: The Organization; 2009.
- Kampf G, Ostermeyer C. World Health Organization-recommended hand-rub formulations do not meet European efficacy requirements for surgical hand disinfection in five minutes. J Hosp Infect. 2011;78:123–7. DOIPubMedGoogle Scholar
- European Committee for Standardization. European Norm (EN) 12791. Chemical disinfectants and antiseptics. Surgical hand disinfection—test method and requirement (phase 2/step 2). Brussels: The Committee; 2018.
- Suchomel M, Kundi M, Pittet D, Weinlich M, Rotter ML. Testing of the World Health Organization recommended formulations in their application as hygienic hand rubs and proposals for increased efficacy. Am J Infect Control. 2012;40:328–31. DOIPubMedGoogle Scholar
- European Committee for Standardization. European Norm (EN) 1500. Chemical disinfectants and antiseptics—hygienic hand disinfection—test method and requirement (phase 2/step 2). Brussels: The Committee; 2018.
- Suchomel M, Kundi M, Allegranzi B, Pittet D, Rotter ML. Testing of the World Health Organization-recommended formulations for surgical hand preparation and proposals for increased efficacy. J Hosp Infect. 2011;79:115–8. DOIPubMedGoogle Scholar
- Suchomel M, Rotter M, Weinlich M, Kundi M. Glycerol significantly decreases the three hour efficacy of alcohol-based surgical hand rubs. J Hosp Infect. 2013;83:284–7. DOIPubMedGoogle Scholar
- Suchomel M, Kundi M, Pittet D, Rotter ML. Modified World Health Organization hand rub formulations comply with European efficacy requirements for preoperative surgical hand preparations. Infect Control Hosp Epidemiol. 2013;34:245–50. DOIPubMedGoogle Scholar
- Menegueti MG, Laus AM, Ciol MA, Auxiliadora-Martins M, Basile-Filho A, Gir E, et al. Glycerol content within the WHO ethanol-based handrub formulation: balancing tolerability with antimicrobial efficacy. Antimicrob Resist Infect Control. 2019;8:109. DOIPubMedGoogle Scholar
- Allegranzi B, Aiken AM, Zeynep Kubilay N, Nthumba P, Barasa J, Okumu G, et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: a multicentre, before-after, cohort study. Lancet Infect Dis. 2018;18:507–15. DOIPubMedGoogle Scholar
- Fluhr JW, Darlenski R, Surber C. Glycerol and the skin: holistic approach to its origin and functions. Br J Dermatol. 2008;159:23–34. DOIPubMedGoogle Scholar
Page created: May 27, 2020
Page updated: August 18, 2020
Page reviewed: August 18, 2020
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.