Volume 27, Number 7—July 2021
Research
Ethnically Disparate Disease Progression and Outcomes among Acute Rheumatic Fever Patients in New Zealand, 1989–2015
Abstract
We investigated outcomes for patients born after 1983 and hospitalized with initial acute rheumatic fever (ARF) in New Zealand during 1989–2012. We linked ARF progression outcome data (recurrent hospitalization for ARF, hospitalization for rheumatic heart disease [RHD], and death from circulatory causes) for 1989–2015. Retrospective analysis identified initial RHD patients <40 years of age who were hospitalized during 2010–2015 and previously hospitalized for ARF. Most (86.4%) of the 2,182 initial ARF patients did not experience disease progression by the end of 2015. Progression probability after 26.8 years of theoretical follow-up was 24.0%; probability of death, 1.0%. Progression was more rapid and ≈2 times more likely for indigenous Māori or Pacific Islander patients. Of 435 initial RHD patients, 82.2% had not been previously hospitalized for ARF. This young cohort demonstrated low mortality rates but considerable illness, especially among underserved populations. A national patient register could help monitor, prevent, and reduce ARF progression.
Acute rheumatic fever (ARF) is a rare inflammatory condition triggered in response to untreated group A Streptococcus infection. ARF rates peak among children 5–14 years of age (1). ARF may permanently damage cardiac valves, producing chronic rheumatic heart disease (RHD), a serious, sometimes fatal, condition that may require surgery (2). Approximately half the children who experience an initial episode of ARF sustain cardiac damage, which persists as RHD for ≈15%–50% (3). Repeated ARF attacks (recurrent ARF) can produce new, and worsen existing, cardiac damage. If long-term prophylaxis (intramuscular injections of benzathine penicillin G [BPG]) is not administered regularly, ≈50% of ARF patients will experience recurrent ARF (4). Secondary prophylaxis is complicated by access to healthcare, cultural appropriateness of care delivery, injection-related discomfort, and health literacy. RHD can also develop without any previously recognized ARF (5,6). The World Health Organization recommends establishing patient registers to assist with best-practice patient management in areas where ARF persists. New Zealand lacks a national ARF register, despite a significant disease burden (7).
In most high-income countries, ARF is rare; rates declined sharply from the 1960s. This decline is largely attributed to improved socioeconomic and living conditions that reduce group A Streptococcus infections and to increased use of antimicrobial drugs to treat infections before ARF onset (1,8–10). Pacific Islanders make up 7% of the New Zealand population; migration between New Zealand and other Pacific Island countries occurs regularly (11). ARF rates for indigenous Australian, New Zealand Māori, and Pacific Islander populations are among the highest in the world (12,13). In New Zealand, deaths from ARF are uncommon, but RHD causes ≈140 deaths and 600 hospitalizations annually; Māori and Pacific Islander persons are overrepresented (14).
In New Zealand, the National Health Index number (NHI), a unique identifier, can identify and link a person’s information across health datasets. However, information regarding the extent to which ARF patients experience poor health outcomes is limited (15). Patient register data (which includes echocardiographic records) from Northern Territory, Australia, show that RHD developed within 10 years of a new ARF diagnosis for 61% of indigenous patients (16). In New Zealand, ARF is legally notifiable; however, considerable historic undernotification impairs the usefulness of surveillance data. Thus, epidemiologic analyses often rely on hospital admission data in the national minimum dataset (NMDS), which contains data on all publicly funded hospitalizations. The NDMS is affected by misdiagnosis and miscoding and is estimated to be 80% sensitive for detecting true ARF patients (17). NMDS specificity for identifying RHD is also an issue. Historically, patients who have valve disease without known cause were assigned International Classification of Diseases (ICD) codes for RHD (18). Analyses of ICD codes for RHD can thus overestimate true cases, particularly in high-income countries, where as few as 32% of patients assigned RHD codes have genuine probable/possible RHD (19). The ARF diagnosis can be complex and easy for clinicians to miss (2). Mild-to-moderate RHD may not necessitate hospital admission, and outpatient records are not compiled on a national level. Although it is recommended that persons with initial or recurrent ARF are hospitalized for optimal management (2), adult patients with minimal or no symptoms are often reluctant to be admitted. These issues make evaluating ARF prevention and control activities challenging (7).
The prognosis for patients with subclinical RHD is unclear. These patients may not experience clinically apparent ARF but rather experience cardiac changes consistent with RHD, detectable using echocardiography only. Without prophylaxis, some patients may experience further cardiac damage eventually resulting in clinically evident RHD. Therefore, echocardiographic screening of high-risk children to identify subclinical RHD cases and provide prophylactic treatment/monitoring may be needed to effectively reduce the RHD burden (20,21).
Given the absence of a national patient register from which to monitor New Zealand ARF patient outcomes, our first aim was to quantify the proportion of patients with initial ARF who progressed to hospitalization with recurrent ARF or RHD or died from circulatory causes (circulatory death) and to investigate their risk for disease progression according to selected demographic and clinical characteristics. Our second aim was to determine the proportion of patients with initial RHD who were hospitalized with previous ARF. Ethics approval was provided by the University of Otago Human Ethics Committee (HD 17/452), including a waiver of consent to use deidentified health data.
Aim 1: Determining Progression of Initial ARF to Recurrent ARF, RHD Hospitalization, and Early Death
In New Zealand, NMDS data with universal use of the NHI are available from 1988 on (22).; we extracted hospital admission data for 1989–2015. We extracted mortality data for 1989–2015 from the national Mortality Collection, which classifies the underlying cause of death for all registered deaths (23). We excluded from analysis non–New Zealand residents and all hospital transfers.
RHD Dataset
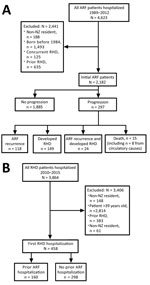
We extracted NMDS data for patients hospitalized with RHD for the first time during 1989–2015 (Figure 1, Initial RHD). These patients had not previously received a diagnosis of RHD or a concurrent diagnosis of ARF.
Initial ARF Dataset
We extracted NMDS data for patients who were hospitalized and assigned a principal diagnosis of ARF during 1989–2012 (Figure 1, Initial ARF). To maximize data accuracy and completeness, we excluded patients born before January 1, 1984. Included patients would therefore have been <5 years of age at the start of the study period. Because ARF is very rare in children <4 years of age, all ARF hospitalizations would be captured in this cohort (24,25). To increase the average follow-up time, we excluded patients hospitalized for initial ARF after December 31, 2012. Consequently, the oldest possible participant age by the end of the follow-up period (December 31, 2015) was 31 years and the youngest possible age was 3 years.
We excluded persons who had concurrent RHD and initial ARF (Figure 2, panel A). Concurrent cases were identified when an encrypted NHI corresponding to an initial ARF hospitalization was matched to the RHD dataset and both hospitalizations occurred within 180 days of each other. The 180-day cutoff point was selected by using clinical advice from a pediatric cardiologist experienced in treating ARF and RHD. A data subset of initial ARF patients was created, as was a data subset of concurrent cases. Patients were considered to have had carditis if ICD codes 101, 102, 1020, 391, 392, or 3920 were listed with their initial ARF hospitalization.
Recurrent ARF Dataset
The encrypted NHI identified all repeated hospitalizations occurring within 180 days of each other for which ARF was the principal diagnosis during 1989–2015 (Figure 1, Recurrent ARF). A data subset for patients with recurrent ARF was created (Figure 2, panel A).
RHD Progression Dataset
We used the encrypted NHI to match persons in the initial ARF dataset with the RHD dataset (Figure 1, Progression to Initial RHD). We created a data subset of patients with initial ARF that progressed to hospitalization for RHD (Figure 2, panel A).
ARF Mortality Datasets
We used the encrypted NHI to match the initial ARF dataset with the Mortality Collection. When a match was made, we extracted the date and cause of death. We identified initial ARF patients who died before January 1, 2016. We noted when the primary cause of death was attributed to diseases of the circulatory system (Figure 1, Circulatory Death; codes 390–459 from ICD 9th Revision, 100–199 ICD 10th Revision). We created a data subset of initial ARF patients who died from circulatory causes (Figure 2, panel A).
Any Progression Dataset
We combined data subsets of patients with initial ARF who progressed to hospitalization with recurrent ARF or RHD, to circulatory death, or both (Figure 1, Progression [Any]). The resulting dataset identified initial ARF patients who experienced disease progression before January 1, 2016. We tabulated key demographic and clinical characteristics of patients who did and did not progress.
Aim 2: Determining Proportion of RHD Patients with Previous ARF
Initial RHD patients were identified in NMDS data when an ICD code corresponding to RHD (Figure 1, Initial RHD) was applied for the first time as a principal diagnosis during January 1, 2010–December 31, 2015. To maximize chances of detecting the first hospitalization with ARF/RHD as a primary diagnosis, we excluded RHD patients >39 years of age. We applied inclusion and exclusion criteria when identifying initial RHD patients who had and had not been hospitalized with previous ARF (Figure 2, panel B).
We used the 180-day separation to distinguish ARF progression from patients who concurrently had ARF and RHD (aim 1) and from patients with multiple ARF hospitalizations for their first ARF episode (aim 2). When observing ARF progression, patients with ICD code(s) corresponding to initial RHD as principal diagnosis <180 days from their initial ARF hospitalization were classified as having concurrent ARF and RHD (aim 1). When RHD preceded ARF, patients with diagnostic code(s) corresponding to ARF applied as principal diagnosis <180 days of their initial RHD hospitalization were classified as having concurrent ARF and RHD (aim 2).
Statistical Analyses
We used R software version 3.1.0 throughout our analysis (26). Demographic data analyzed included patient age at hospitalization, New Zealand resident status, sex, prioritized ethnicity, and 2006/2013 New Zealand Deprivation Index (NZDep06/NZDep13), all of which were encoded by the NHI. Prioritized ethnicity identifies persons belonging to multiple ethnic groups and reallocates a single ethnic group by using a prioritized order of Māori, Pacific Islander, Asian, and other (27). The NZDep06/NZDep13 classification system measures socioeconomic deprivation in small geographic areas by using census data (28). Quintile 1 represents persons living in the least deprived neighborhoods; quintile 5, the most deprived neighborhoods.
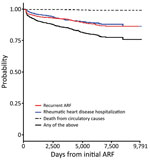
To investigate whether reported proportions differed significantly between groups, we used the χ2 test. We used the Mann-Whitney U test to compare differences in progression time from initial ARF hospitalization to RHD progression (aim 1) and time from preceding ARF to initial RHD hospitalization (aim 2). We used Kaplan-Meier modeling to estimate the probability of disease progression over a theoretical 9,791-day (i.e., 26.8-year) follow-up period by extrapolating observed progression rates. This period was the maximum time that any person in the dataset was observed. Outcomes were investigated individually and together as the “any progression” group. Observations were right censored at the end of the study period.
Generalized linear models calculated odds ratios (ORs) and 95% CIs of progression outcomes by selected characteristics. Cox-proportional hazard ratios (HRs) and 95% CIs described whether initial ARF patients with certain characteristics tended to experience disease progression sooner than others. We considered p<0.05 to be significant.
Aim 1: Study Population
During 1989–2012, a total of 4,623 ARF patients were hospitalized with ARF for the first time; 2,182 met the inclusion criteria (Figure 2, panel A). The median follow-up time for this cohort was 10.4 years (range 3.0–26.8 years, interquartile range [IRQ] 6.4–15.3 years). Most initial ARF patients were 5–14 years of age (83.3%), male (57.9%), and of Māori (54.4%) or Pacific Islander (36.4%) ethnicity. Most (66.9%) were from NZDep06 quintile 5 (the most deprived neighborhoods). Just over half (51.9%) had carditis (Table 1).
Of the initial ARF patients hospitalized for RHD, 42% (125/298) had RHD concurrently and were excluded (aim 1). Similarly, of the initial RHD patients who experienced ARF, 46% (65/142) had concurrent ARF (aim 2) and were excluded. The time distribution to progression supports use of the 180-day cutoff (Appendix Figure 1, panels A, B).
Aim 1A: Risk for Progression from Initial ARF to Recurrent ARF or RHD Hospitalization and Early Death
A total of 297 (13.6%) of the 2,182 patients with initial ARF experienced disease progression before January 1, 2016. Of these, 142 (6.5%) were hospitalized with recurrent ARF and 173 (7.9%) with RHD; 24 were hospitalized for both. Fifteen initial ARF patients died, 8 from circulatory causes (Figure 2, panel A).
The median time from initial ARF to recurrent ARF hospitalization was 3.2 years (IQR 1.9–8.4 years) and to RHD hospitalization was 4.0 years (IQR 1.9–8.4 years). The median time to circulatory death was 10.4 years (IQR 3.3–12.8 years).
The overall probability of experiencing disease progression (to hospitalization with recurrent ARF/RHD or to circulatory death) within a theoretical 9,791 days from the initial ARF hospitalization was 24.0%. When progression outcomes were considered individually, the probability of recurrent ARF hospitalization was 23.5%, as was the probability of being hospitalized for RHD. The risk for death was low: 1.0% (Figure 3).
Aim 1B: Risk Factors for Progression from Initial ARF
Risk for disease progression was higher for Māori (OR 1.68, 95% CI 1.10–2.67) and Pacific Islander (OR 2.12, 95% CI 1.37–3.39) patients than for persons of European or other ethnicities. Progression occurred sooner for Māori (HR 1.89, 95% CI 1.24–2.88) and Pacific Islander (HR 2.35, 95% CI 1.54–3.60) patients. Disease progression was twice as likely for patients with carditis (OR 2.00, 95% CI 1.57–2.54) than without carditis, and progression occurred sooner (HR 1.94, 95% CI 1.55–2.43). We noted no significant differences in risk for disease progression by sex, age, or NZDep 06 quintile (Table 2). No factors in Table 2 were found to be significant predictors of recurrent ARF.
Risk for disease progression to RHD hospitalization was higher for Māori (OR 2.09, 95% CI 1.09–4.52) and Pacific Islander (OR 3.64, 95% CI 1.91–7.86) patients than for patients of European or other ethnicities and occurred sooner (HR 2.54, 95% CI 1.27–5.10 for Māori; HR 2.53, 95% CI 1.27–5.05 for Pacific Islanders). Initial ARF patients with carditis were more likely to experience RHD (OR 5.19, 95% CI 3.52–7.89) than those without carditis. Patients with initial ARF whose condition progressed to recurrent ARF were more likely to experience progression to hospitalization for RHD than patients who did not experience recurrent ARF (OR 3.10, 95% CI 2.07-4.55). Small patient numbers meant that no factors predicted circulatory death, with the exception of carditis (OR 6.52, 95% CI 1.16–122.00; Appendix Table 2).
Aim 2: Proportion of Initial RHD Patients with Preceding ARF
A total of 3,836 patients were hospitalized with RHD during 2010–2015; of these, 435 patients with initial RHD met the inclusion criteria (Figure 2, panel B), 102 of whom were also included in the initial ARF dataset for aim 1. Most patients were female (229, 52.6%), Pacific Islander (207, 47.6%), or Māori (176, 40.5%) and were from the most deprived neighborhoods (271 [62.3%] NZDep13 quintile 5). Previous hospitalization for ARF (i.e., >180 days before initial RHD hospitalization) was detected for 77 patients (17.8%; Figure 2, panel B). Of the 335 initial RHD patients <30 years of age, 19.4% had been previously hospitalized for ARF.
Of the Māori patients, 21.6% were previously hospitalized for ARF, as were 18.4% of Pacific Islander patients. A significantly lower proportion (1.9%) of patients of European and other ethnicities were previously hospitalized for ARF (p<2.2 × 10–16). A lower proportion of female patients (11.4%) were previously hospitalized for ARF than were male patients (24.8%; p = 2.565 × 10–6). Of the patients from NZDep quintile 5, a total of 19.9% were previously hospitalized for ARF, as were 15.4% of patients from other quintiles (p = 0.048; Appendix Table 1). There was no difference in overall time of progression from initial ARF to RHD hospitalization compared with time from initial RHD going back to preceding ARF hospitalization (p˃0.05; Appendix Figure 1).
This study demonstrates concerning ethnic inequities in ARF progression. By the end of the study period, 14% of initial ARF patients (with no concurrent RHD) had experienced progression to recurrent ARF, RHD, or circulatory death. However, ARF progression was approximately twice as likely for Māori and Pacific Islander patients and occurred approximately twice more rapidly. It is concerning that of 435 initial RHD patients <40 years of age, <1 in 5 were hospitalized with preceding ARF, severely limiting opportunities for secondary prevention. Ethnic inequities in ARF progression add to extreme ethnic inequities in the burden of ARF (24,25). Possible reasons for increased illness among Māori and Pacific Islander patients include the inequitable distribution of the underlying determinants of health, such as access to health services, nutrition, and a healthy home environment (29,30). Genetic and immunologic factors may also contribute (30–33). Similar findings have been reported for indigenous patients in Australia (15).
That 14% of the initial ARF cohort experienced progression suggests failures in secondary prophylaxis to which Māori and Pacific Islander patients may experience barriers. We support creating a national patient register by drawing on data from regional registers. The goal would be to improve secondary prophylaxis uptake by coordinating treatment for patients who are frequently mobile. There is widespread support for a national register among stakeholders, which could be expanded to monitor patients’ RHD status and health outcomes (7,34). Previous attempts to set up a national register have failed because of privacy concerns. A perceived lack of political will to implement such a register has been noted (35,36).
Disease progression for New Zealand ARF patients overall seems to be considerably less than that reported for indigenous patients in Australia (16). This difference probably reflects multiple factors, including difficulty delivering consistent medical treatment in remote areas and use of echocardiography outreach clinics in Australia (which may detect RHD sooner than when signs/symptoms otherwise come to medical attention) (16,37,38). Our findings may be specific to New Zealand.
Although we did not identify differences in ARF progression by sex, these differences have been reported elsewhere (16,39). Our finding that 12% of female patients with initial RHD were previously hospitalized with ARF, compared with 25% of male patients, may suggest that clinical manifestations and outcomes for female patients warrant investigation.
More than 80% of young initial RHD patients had not been previously hospitalized for ARF, which indicates that many ARF patients do not come to clinical attention and miss prophylactic treatment. Increasing public and clinician awareness of ARF, echocardiographic screening for high-risk children, and new diagnostic tools may improve case identification (21,40). If a clear prognostic benefit is demonstrated from echocardiography screening programs, this finding may strongly support the use of targeted screening among high-risk New Zealand children. It is unlikely that RHD detected through echocardiography screening studies would have affected this analysis because they would receive outpatient assessment (41).
The reliance on hospital data is a major limitation of, and justification for, this study. In New Zealand, gaps in data completeness for ARF/RHD are closing; however, the study period is affected (42). Some patients may have been inappropriately included or missed from this analysis, or progression outcomes may be misclassified. This study markedly underestimates the proportion of ARF patients whose condition will ultimately progress because of limited follow-up time; furthermore, hospitalization data do not capture outpatients (2). Although a prospective study design would enable a more nuanced analysis of ARF progression, severe outcomes may take many years to develop (43). Repeat analyses of this study cohort will provide a more complete assessment of progression risk. Migration of RHD patients into New Zealand may account for some occurrences where no preceding ARF hospitalization was identified (44). The high (94%) proportion of children <10 years of age with initial RHD and no preceding/concurrent ARF hospitalization may result from miscoding (with ARF incorrectly coded as RHD; Appendix Table 1). Use of the >180-day window was supported when examining time intervals to progression (Appendix) and by the small number of studies reporting on ARF progression (45,46). A quality systematic patient audit would be valuable for assessing the validity of diagnostic coding. It would also be useful to audit a sample of initial RHD patients not previously hospitalized for ARF to see if diagnostic opportunities had been missed.
A key study strength is use of the encrypted NHI to identify persons within and between datasets, which permits inclusion of an entire national cohort. Hospitalization is the standard of care for all patients with suspected initial ARF in New Zealand (2). Ambulatory care data and prophylaxis data were not available (2). Published data on BPG adherence are inconsistently available. A regional study of 77 ARF patients identified 51% as fully adherent to BPG prophylaxis (47). An audit from Auckland (where ≈50% of patients reside) indicated that ≈96% of ARF patients were fully adherent in the 2 largest regions, but adherence fell from 93% in 1998 to 86% in 2000 in the smaller (Waitemata) region (48). The extent to which progression rates were affected by ARF patients’ adherence to secondary prophylaxis in this analysis is unknown.
In summary, our study better defines ARF disease progression in New Zealand. After their initial ARF hospitalization, 14% of patients were hospitalized with recurrent ARF or RHD or died; that proportion will probably increase over time. Māori and Pacific Islander patients face an increased risk for ARF progression. Four fifths of initial RHD patients had no preceding ARF hospitalization recorded, thus limiting opportunities for prophylaxis. The need to enhance clinical care delivery for underserved groups is strongly indicated. A national patient register may improve prophylaxis uptake, clinical service coordination, and sector performance monitoring. Further research into echocardiography screening is needed. Our results show a clear need to address the major modifiable determinants of health and equity; ARF and RHD represent indicators of progress that should be closely monitored.
Dr. Oliver is a postdoctoral research fellow and works as infectious disease researcher at the University of Melbourne and Murdoch Children’s Research Institute. Her research focus is streptococcal diseases and Buruli ulcer. This study formed part of her doctoral thesis, which concerns acute rheumatic fever, its risk factors, and determinants.
Acknowledgments
We acknowledge Janine Ryland, who was a member of Brooke Marsters’ advisory group.
This work was supported by a grant from the New Zealand Lotteries Health Commission, in the form of a PhD scholarship awarded to J.O. and a Health Research Council of New Zealand project grant providing support for M.G.B. and J.B. B.M. was supported by a Pacific Health Research Summer Studentship from the Health Research Council of New Zealand. D.A.W. is supported by a Fellowship from the National Health and Medical Research Council of Australia (GNT 1123854).
References
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5:685–94. DOIPubMedGoogle Scholar
- Heart Foundation of New Zealand. New Zealand guidelines for rheumatic fever: diagnosis, management and secondary prevention of acute rheumatic fever and rheumatic heart disease: 2014 update. Auckland (New Zealand); The Foundation; 2014.
- Caldas AM, Terreri MTA, Moises VA, Silva CM, Len CA, Carvalho AC, et al. What is the true frequency of carditis in acute rheumatic fever? A prospective clinical and Doppler blind study of 56 children with up to 60 months of follow-up evaluation. Pediatr Cardiol. 2008;29:1048–53. DOIPubMedGoogle Scholar
- Porth C. Essentials of pathophysiology: concepts of altered health states. Hagerstown (MD): Lippincott Williams & Wilkins; 2007.
- Carapetis JR, Currie BJ, Good MF. Towards understanding the pathogenesis of rheumatic fever. Scand J Rheumatol. 1996;25:127–31, discussion 132–3. DOIPubMedGoogle Scholar
- Gemechu T, Mahmoud H, Parry EH, Phillips DI, Yacoub MH. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. Eur J Prev Cardiol. 2017;24:717–23. DOIPubMedGoogle Scholar
- Oliver J, Pierse N, Baker MG. Improving rheumatic fever surveillance in New Zealand: results of a surveillance sector review. BMC Public Health. 2014;14:528. DOIPubMedGoogle Scholar
- Nkomo VT. Epidemiology and prevention of valvular heart diseases and infective endocarditis in Africa. Heart. 2007;93:1510–9. DOIPubMedGoogle Scholar
- Rizvi SF, Khan MA, Kundi A, Marsh DR, Samad A, Pasha O. Status of rheumatic heart disease in rural Pakistan. Heart. 2004;90:394–9. DOIPubMedGoogle Scholar
- Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart. 2013;99:1554–61. DOIPubMedGoogle Scholar
- Statistics NZ. Pacific Peoples ethnic group [cited 2018 Feb 14]. http://archive.stats.govt.nz/Census/2013-census/profile-and-summary-reports/quickstats-culture-identity/pacific-peoples.aspx
- Ralph AP, Carapetis JR. Group A streptococcal diseases and their global burden. In: Chhatwal GS, editor. Host-pathogen interactions in streptococcal diseases. Berlin: Springer-Verlag; 2013. p. 1–27.
- Oliver J, Upton A, Jack SJ, Pierse N, Williamson DA, Baker MG. Distribution of streptococcal pharyngitis and acute rheumatic fever, Auckland, New Zealand, 2010–2016. Emerg Infect Dis. 2020;26:1113–21. DOIPubMedGoogle Scholar
- Bennett J, Zhang J, Leung W, Jack S, Oliver J, Webb R, et al. Rising ethnic inequalities in acute rheumatic fever and rheumatic heart disease, New Zealand, 2000–2018. Emerg Infect Dis. 2021;27:27. DOIPubMedGoogle Scholar
- He VY, Condon JR, Ralph AP, Zhao Y, Roberts K, de Dassel JL, et al. Long-term outcomes from acute rheumatic fever and rheumatic heart disease: a data-linkage and survival analysis approach. Circulation. 2016;134:222–32. DOIPubMedGoogle Scholar
- Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013;128:492–501. DOIPubMedGoogle Scholar
- Oliver J, Pierse N, Baker MG. Estimating rheumatic fever incidence in New Zealand using multiple data sources. Epidemiol Infect. 2015;143:167–77. DOIPubMedGoogle Scholar
- Fitz-Gerald JA, Ongzalima CO, Ng A, Greenland M, Sanfilippo FM, Hung J, et al. A validation study: how predictive is a diagnostic coding algorithm at identifying rheumatic heart disease in Western Australian hospital data? Heart Lung Circ. 2019.PubMedGoogle Scholar
- Katzenellenbogen JM, Nedkoff L, Cannon J, Kruger D, Pretty F, Carapetis JR, et al. Low positive predictive value of International Classification of Diseases, 10th Revision codes in relation to rheumatic heart disease: a challenge for global surveillance. Intern Med J. 2019;49:400–3. DOIPubMedGoogle Scholar
- Beaton A, Aliku T, Dewyer A, Jacobs M, Jiang J, Longenecker CT, et al. Latent rheumatic heart disease: identifying the children at highest risk of unfavorable outcome. Circulation. 2017;136:2233–44. DOIPubMedGoogle Scholar
- Engelman D, Mataika RL, Ah Kee M, Donath S, Parks T, Colquhoun SM, et al. Clinical outcomes for young people with screening-detected and clinically-diagnosed rheumatic heart disease in Fiji. Int J Cardiol. 2017;240:422–7. DOIPubMedGoogle Scholar
- Ministry of Health. National minimum dataset (hospital events) [cited 2012 Sep 13]. https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/national-minimum-dataset-hospital-events
- New Zealand Ministry of Health. Mortality Collection data dictionary [cited 2019 Mar 20]. https://www.health.govt.nz/system/files/documents/publications/mortality_data_dictionary_v1.6_final.pdf
- Milne RJ, Lennon DR, Stewart JM, Vander Hoorn S, Scuffham PA. Incidence of acute rheumatic fever in New Zealand children and youth. J Paediatr Child Health. 2012;48:685–91. DOIPubMedGoogle Scholar
- Jaine R, Baker M, Venugopal K. Epidemiology of acute rheumatic fever in New Zealand 1996-2005. J Paediatr Child Health. 2008;44:564–71. DOIPubMedGoogle Scholar
- R Core Team. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2014.
- Ministry of Health. Ethnicity data protocols for the health and disability sector. Wellington (New Zealand); The Ministry; 2004.
- Salmond CE, Crampton P. Development of New Zealand’s deprivation index (NZDep) and its uptake as a national policy tool. Can J Public Health. 2012;103(Suppl 2):S7–11.PubMedGoogle Scholar
- Hobbs M, Tomintz M, McCarthy J, Marek L, Vannier C, Campbell M, et al. Obesity risk in women of childbearing age in New Zealand: a nationally representative cross-sectional study. Int J Public Health. 2019;64:625–35. DOIPubMedGoogle Scholar
- Baker MG, Gurney J, Oliver J, Moreland NJ, Williamson DA, Pierse N, et al. Risk factors for acute rheumatic fever: literature review and protocol for a case-control study in New Zealand. Int J Environ Res Public Health. 2019;16:
E4515 . DOIPubMedGoogle Scholar - Hill S, Sarfati D, Robson B, Blakely T. Indigenous inequalities in cancer: what role for health care? ANZ J Surg. 2013;83:36–41. DOIPubMedGoogle Scholar
- Cheng TO. How much of the recent decline in rheumatic heart disease in China can be explained by changes in cardiovascular risk factors? Int J Cardiol. 2009;132:300–2. DOIPubMedGoogle Scholar
- Goodman A, Kajantie E, Osmond C, Eriksson J, Koupil I, Thornburg K, et al. The relationship between umbilical cord length and chronic rheumatic heart disease: a prospective cohort study. Eur J Prev Cardiol. 2015;22:1154–60. DOIPubMedGoogle Scholar
- Anderson A, Peat B, Ryland J, Ofanoa M, Burgess H, Malungahu G, et al. Mismatches between health service delivery and community expectations in the provision of secondary prophylaxis for rheumatic fever in New Zealand. Aust N Z J Public Health. 2019;43:294–9. DOIPubMedGoogle Scholar
- Thornley C, McNicholas A, Baker M, Lennon D. Rheumatic fever registers in New Zealand. New Zealand Public Health Report. 2001;8:41–4.
- Oliver J, Pierse N, Baker MG. Improving rheumatic fever surveillance in New Zealand: results of a surveillance sector review. BMC Public Health. 2014;14:528. DOIPubMedGoogle Scholar
- Anderson RD, Pepine CJ. Gender differences in the treatment for acute myocardial infarction: bias or biology? Circulation. 2007;115:823–6. DOIPubMedGoogle Scholar
- McDonald M, Towers RJ, Andrews RM, Carapetis JR, Currie BJ. Epidemiology of Streptococcus dysgalactiae subsp. equisimilis in tropical communities, Northern Australia. Emerg Infect Dis. 2007;13:1694–700. DOIPubMedGoogle Scholar
- Ministry of Health. Cardiovascular disease (50+ years) [cited 2017 Nov 3]. https://www.health.govt.nz/nz-health-statistics/health-statistics-and-data-sets/Māori-health-data-and-stats/tatau-kura-tangata-health-older-Māori-chart-book/nga-mana-hauora-tutohu-health-status-indicators-50-years/cardiovascular-disease-50-years
- Gauld R, Horsburgh S. Does a host country capture knowledge of migrant doctors and how might it? A study of UK doctors in New Zealand. Int J Public Health. 2016;61:1–8. DOIPubMedGoogle Scholar
- Webb RH, Wilson NJ, Lennon DR, Wilson EM, Nicholson RW, Gentles TL, et al. Optimising echocardiographic screening for rheumatic heart disease in New Zealand: not all valve disease is rheumatic. Cardiol Young. 2011;21:436–43. DOIPubMedGoogle Scholar
- Oliver J, Pierse N, Williamson DA, Baker MG. Estimating the likely true changes in rheumatic fever incidence using two data sources. Epidemiol Infect. 2018;146:265–75. DOIPubMedGoogle Scholar
- Milne RJ, Lennon D, Stewart JM, Vander Hoorn S, Scuffham PA. Mortality and hospitalisation costs of rheumatic fever and rheumatic heart disease in New Zealand. J Paediatr Child Health. 2012;48:692–7. DOIPubMedGoogle Scholar
- Darlington-Pollock F, Shackleton N, Norman P, Lee AC, Exeter D. Differences in the risk of cardiovascular disease for movers and stayers in New Zealand: a survival analysis. Int J Public Health. 2018;63:173–9. DOIPubMedGoogle Scholar
- Voss LM, Wilson NJ, Neutze JM, Whitlock RM, Ameratunga RV, Cairns LM, et al. Intravenous immunoglobulin in acute rheumatic fever: a randomized controlled trial. Circulation. 2001;103:401–6. DOIPubMedGoogle Scholar
- Group CRFS. The treatment of acute rheumatic fever in children. A cooperative clinical trial of ACTH, cortisone and aspirin. Circulation. 1955;11:343–77. DOIGoogle Scholar
- Oetzel JG, Lao C, Morley M, Penman K, Child M, Scott N, et al. Efficacy of an incentive intervention on secondary prophylaxis for young people with rheumatic fever: a multiple baseline study. BMC Public Health. 2019;19:385. DOIPubMedGoogle Scholar
- Grayson S, Horsburgh M, Lennon D. An Auckland regional audit of the nurse-led rheumatic fever secondary prophylaxis programme. N Z Med J. 2006;119:U2255.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 07, 2021
Table of Contents – Volume 27, Number 7—July 2021
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Jane Oliver, University of Melbourne, 792 Elizabeth St, Melbourne, VIC 3000, Australia
Top