Volume 29, Number 10—October 2023
Research
Candida auris Clinical Isolates Associated with Outbreak in Neonatal Unit of Tertiary Academic Hospital, South Africa
Figure 5
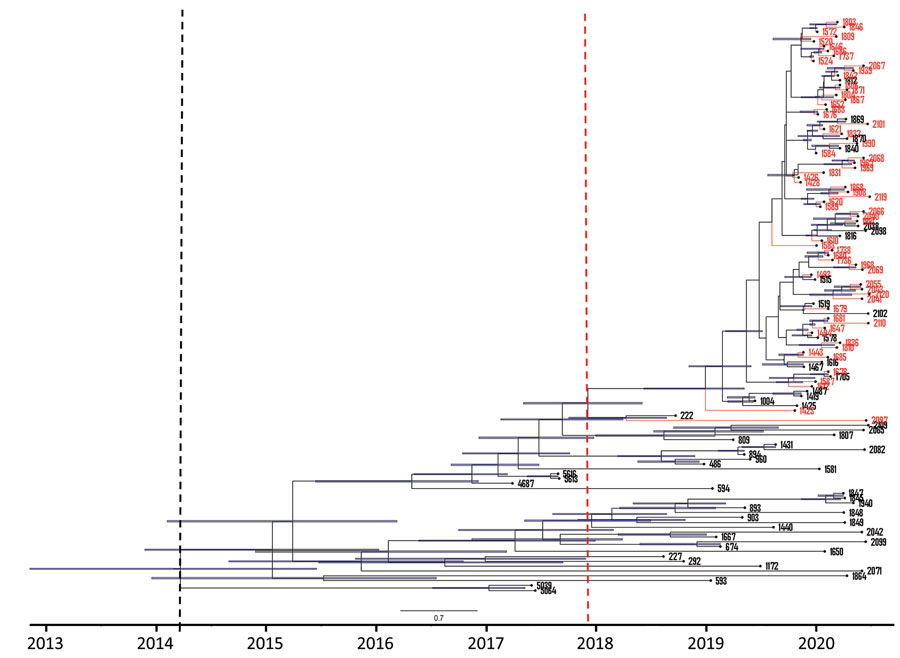
Figure 5. Maximum clade credibility tree of 118 South Africa clade III Candida auris isolates from patients at an academic tertiary hospital in South Africa estimated using BEAST strict clock and coalescent model (24). Red tips represent cases from the neonatal ward, blue bars represent 95% highest probability density black dashed line indicates clade III tMRCA, and red dashed line indicates outbreak strain tMRCA. tMRCA, time to most recent common ancestor.
References
- Spivak ES, Hanson KE. Candida auris: an emerging fungal pathogen. J Clin Microbiol. 2018;56:e01588–17. DOIPubMedGoogle Scholar
- Sardi JCO, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJS. Candida species: current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62:10–24. DOIPubMedGoogle Scholar
- Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol. 2009;53:41–4. DOIPubMedGoogle Scholar
- Govender NP, Patel J, Magobo RE, Naicker S, Wadula J, Whitelaw A, et al.; TRAC-South Africa group. Emergence of azole-resistant Candida parapsilosis causing bloodstream infection: results from laboratory-based sentinel surveillance in South Africa. J Antimicrob Chemother. 2016;71:1994–2004. DOIPubMedGoogle Scholar
- Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2017;64:134–40. DOIPubMedGoogle Scholar
- World Health Organization. WHO fungal priority pathogens list to guide research, development and public health action. 2022 Oct 25 [cited 2023 Jan 15]. https://www.who.int/publications/i/item/9789240060241
- van Schalkwyk E, Mpembe RS, Thomas J, Shuping L, Ismail H, Lowman W, et al.; GERMS-SA. GERMS-SA. Epidemiologic shift in candidemia driven by Candida auris, South Africa, 2016–2017. Emerg Infect Dis. 2019;25:1698–707. DOIPubMedGoogle Scholar
- Govender NP, Avenant T, Brink A, Chibabhai V, Cleghorn J, du Toit B, et al. Federation of Infectious Diseases Societies of Southern Africa guideline: Recommendations for the detection, management and prevention of healthcare-associated Candida auris colonisation and disease in South Africa. S Afr J Infect Dis. 2019;34:163. DOIPubMedGoogle Scholar
- Naicker SD, Maphanga TG, Chow NA, Allam M, Kwenda S, Ismail A, et al. Clade distribution of Candida auris in South Africa using whole genome sequencing of clinical and environmental isolates. Emerg Microbes Infect. 2021;10:1300–8. DOIPubMedGoogle Scholar
- Mashau RC, Meiring ST, Dramowski A, Magobo RE, Quan VC, Perovic O, et al.; Baby GERMS-SA. Culture-confirmed neonatal bloodstream infections and meningitis in South Africa, 2014-19: a cross-sectional study. Lancet Glob Health. 2022;10:e1170–8. DOIPubMedGoogle Scholar
- Shuping L, Mpembe R, Mhlanga M, Naicker SD, Maphanga TG, Tsotetsi E, et al.; for GERMS-SA. for GERMS-SA. Epidemiology of culture-confirmed candidemia among hospitalized children in South Africa, 2012-2017. Pediatr Infect Dis J. 2021;40:730–7. DOIPubMedGoogle Scholar
- Welsh RM, Bentz ML, Shams A, Houston H, Lyons A, Rose LJ, et al. Survival, persistence, and isolation of the emerging multidrug-resistant pathogenic yeast Candida auris on a plastic health care surface. J Clin Microbiol. 2017;55:2996–3005. DOIPubMedGoogle Scholar
- Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, et al. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun. 2018;9:5346. DOIPubMedGoogle Scholar
- Escandón P, Chow NA, Caceres DH, Gade L, Berkow EL, Armstrong P, et al. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis. 2019;68:15–21.PubMedGoogle Scholar
- Maphanga TG, Naicker SD, Kwenda S, Muñoz JF, van Schalkwyk E, Wadula J, et al.; for GERMS-SA. for GERMS-SA. In vitro antifungal resistance of Candida auris isolates from bloodstream infections, South Africa. Antimicrob Agents Chemother. 2021;65:
e0051721 . DOIPubMedGoogle Scholar - Chris Hani Baragwanath Academic Hospital [cited 2022 Sep 10]. https://www.chrishanibaragwanathhospital.co.za
- Berkow EL, Lockhart SR, Ostrosky-Zeichner L. Antifungal susceptibility testing: current approaches. Clin Microbiol Rev. 2020;33:e00069–19. DOIPubMedGoogle Scholar
- Clinical and Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th edition (M27). Wayne (PA): The Institute; 2017.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd edition (M60). Wayne (PA): The Institute; 2020.
- Schmieder R, Edwards R. Quality control and preprocessing of metagenomic datasets. Bioinformatics. 2011;27:863–4. DOIPubMedGoogle Scholar
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–60. DOIPubMedGoogle Scholar
- Sahl JW, Lemmer D, Travis J, Schupp JM, Gillece JD, Aziz M, et al. NASP: an accurate, rapid method for the identification of SNPs in WGS datasets that supports flexible input and output formats. Microb Genom. 2016;2:
e000074 . DOIPubMedGoogle Scholar - Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief Bioinform. 2008;9:299–306. DOIPubMedGoogle Scholar
- Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen). Virus Evol. 2016;2:
vew007 . DOIPubMedGoogle Scholar - Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. DOIPubMedGoogle Scholar
- Choudhuri S. Phylogenetic analysis. In: Bioinformatics for beginners. San Diego: Academic Press; 2014. p. 209–18.
- Frías-De-León MG, Hernández-Castro R, Vite-Garín T, Arenas R, Bonifaz A, Castañón-Olivares L, et al. Antifungal resistance in Candida: molecular determinants. Antibiotics (Basel). 2020;9:1–16. DOIPubMedGoogle Scholar
- Healey KR, Kordalewska M, Jiménez Ortigosa C, Singh A, Berrío I, Chowdhary A, et al. Limited ERG11 mutations identified in isolates of Candida auris directly contribute to reduced azole susceptibility. Antimicrob Agents Chemother. 2018;62:e01427–18. DOIPubMedGoogle Scholar
- Adam RD, Revathi G, Okinda N, Fontaine M, Shah J, Kagotho E, et al. Analysis of Candida auris fungemia at a single facility in Kenya. Int J Infect Dis. 2019;85:182–7. DOIPubMedGoogle Scholar
- Moema I, Ismail H, Van Schalkwyk E, Shuping L, Govender NP. Outbreak of culture-confirmed Candida auris bloodstream infection in the neonatal unit of a public-sector hospital, South Africa, July through September 2017. 2017 [cited 2023 Aug 21]. https://www.tephinet.org/learning/fead/outbreak-culture-confirmed-candida-auris-bloodstream-infection-neonatal-unit-public
- van Schalkwyk E, Iyaloo S, Naicker SD, Maphanga TG, Mpembe RS, Zulu TG, et al. Large outbreaks of fungal and bacterial bloodstream infections in a neonatal unit, South Africa, 2012–2016. Emerg Infect Dis. 2018;24:1204–12. DOIPubMedGoogle Scholar
- Michalski C, Kan B, Lavoie PM. Antifungal immunological defenses in newborns. Front Immunol. 2017;8:281. DOIPubMedGoogle Scholar
- Chow NA, Muñoz JF, Gade L, Berkow EL, Li X, Welsh RM, et al. Tracing the evolutionary history and global expansion of Candida auris using population genomic analyses. MBio. 2020;11:e03364–19. DOIPubMedGoogle Scholar
- Turbett ISE, Becker DSM, Belford MTB, Kelly RTM, Desrosiers MTL, Oliver RNE, et al. Evaluation of Candida auris acquisition in US international travellers using a culture-based screening protocol1. J Travel Med. 2022;29:taab186.
- Chow NA, Gade L, Tsay SV, Forsberg K, Greenko JA, Southwick KL, et al.; US Candida auris Investigation Team. Multiple introductions and subsequent transmission of multidrug-resistant Candida auris in the USA: a molecular epidemiological survey. Lancet Infect Dis. 2018;18:1377–84. DOIPubMedGoogle Scholar
- Yadav V, Heitman J. On fruits and fungi: a risk of antifungal usage in food storage and distribution in driving drug resistance in Candida auris. MBio. 2022;13:
e0073922 . DOIPubMedGoogle Scholar - Miot J, Leong T, Takuva S, Parrish A, Dawood H. Cost-effectiveness analysis of flucytosine as induction therapy in the treatment of cryptococcal meningitis in HIV-infected adults in South Africa. BMC Health Serv Res. 2021;21:305. DOIPubMedGoogle Scholar
- Bravo Ruiz G, Lorenz A. What do we know about the biology of the emerging fungal pathogen of humans Candida auris? Microbiol Res. 2021;242:
126621 . DOIPubMedGoogle Scholar
1Members of GERMS-SA are listed at the end of this article.
Page created: August 31, 2023
Page updated: September 20, 2023
Page reviewed: September 20, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.