Volume 29, Number 12—December 2023
Research Letter
Crimean-Congo Hemorrhagic Fever Virus Seropositivity among Dromedary Camels, Algeria, 2020–2021
Abstract
Serosurvey results for Crimean-Congo hemorrhagic fever virus antibodies in dromedary camels in Algeria indicate that the pathogen is circulating endemically in desertic areas, despite the hostile environment. Thus, dromedaries are suitable sentinels for detecting human risk for Crimean-Congo hemorrhagic fever in desertic areas.
Crimean Congo hemorrhagic fever virus (CCHFV) is a tickborne Orthonairovirus that causes a potentially fatal hemorrhagic systemic disease in humans, Crimean-Congo hemorrhagic fever (CCHF). The virus is sustained in the ecosystem through wild and domestic animals, which act as tick amplification hosts and are asymptomatic (1). CCHF has recently increased in Africa and is emerging in new regions (2). However, knowledge of CCHFV epidemiology in North Africa is limited.
In Algeria, CCHFV has been detected in ticks (3), but no human cases have been reported, probably because of inadequate surveillance (2). In recent years, breeding of dromedary camels (Camelus dromedarius) has increased (4); dromedaries could be ideal indicators of CCHFV circulation because they are widely distributed across Algeria and are commonly reared in open environments with exposure to ticks. To evaluate the distribution of the virus and the potential risk factors associated with CCHFV exposure, we conducted a serosurvey of CCHFV in dromedaries from the northeastern Saharan region of Algeria.
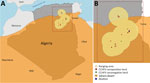
During 2020–2021, we collected 294 serum samples from dromedaries, of which 215 were from 23 different herds, and 79 samples from an abattoir, all from a region that included 4 provinces (wilayas): Biskra, El Oued, Touggourt, and Ouaregla. We tested samples for CCHFV antibodies by using a commercial kit (ID Screen CCHF Double Antigen Multi-species ELISA; IDvet, https://www. id-vet.com). We obtained data on risk factors associated with the individual animals (e.g., age) and management (e.g., breeding system) and evaluated their effect on CCHFV seropositivity with a mixed-effect logistic regression model with herd as a random effect, using R software (The R Project for Statistical Computing, https://www.r-project.org). We also collected data with regard to patterns of movements (i.e., duration and seasonality) for grazing. To evaluate the characteristics of the environment in which the dromedaries were reared, we created a buffer area around each herd (with a 100-km radius, considering the pattern of movements), and obtained the features of the land cover (5).
Animal-level CCHFV seroprevalence was 75.5% (95% CI 69.9–79.8; 222/294), and herd-level seroprevalence was 95.7% (95% CI 93.3–98.0; 22/23) (Figure). Odds of being seropositive for CCHFV were higher among dromedaries bred in the traditional system (i.e., grazing outdoors all year) (7.2, 95% CI 1.1–48.7) and the semitraditional system (i.e., grazing outdoor all year except for winter) (4.5, 95% CI 1.04–19.1) than among animals kept in permanent confinement. Odds of being seropositive were also higher among animals 4–10 years of age (6.5, 95% CI 2.2–19.5) and animals >10 years of age (14.9, 95% CI 3.2–69.4) than among animals <4 years of age (Table). The environment in which the dromedaries grazed was composed essentially of sandy desert (48.0%), bare areas (26.1%), and consolidated bare areas (i.e., bare rocks or stones) (11.5%).
Our results show that exposure of dromedaries to CCHFV is widespread; seroprevalence was high at both the herd and individual animal levels. Individual seroprevalence (74.8%) was similar to that reported in other countries of North Africa (6,7), suggesting that dromedaries may play a role in the epidemiology of CCHFV. Increased age was associated with higher CCHFV seroprevalence, which probably indicates that the virus has been endemic for some years. However, our finding that 22/56 (39.3%) dromedaries <1 year of age were seropositive indicates intense recent CCHFV circulation in the area. Traditional and semitraditional breeding systems increased the likelihood of CCHFV seropositivity because of the increased probability of exposure to CCHFV-infected ticks. Traditionally, dromedaries have been reared in constant movement across large pastoral areas in the Sahara desert, but nomadism is being replaced by transhumance (i.e., shorter and seasonal movements), especially in the northeastern Saharan region of Algeria (8). None of the herds in our study was nomadic; most movements were <4 days, implying that seropositive animals were exposed within the study area. Therefore, CCHFV circulation in dromedaries from this region most likely maintains itself without the need for repeated introductions from neighboring areas.
Attempts to map the distribution of CCHF risk have indicated that the areas at risk in Africa were basically restricted to the sub-Saharan region, where CCHF was associated with the presence of shrub or grassland (9). Because we found that bare or sandy desert areas are also favorable for CCHFV transmission, more studies are needed to evaluate animal hosts and tick vectors involved in CCHFV spread in those areas. Moreover, in the northeastern Saharan region of Algeria, the practice of breeding dromedaries in peri-urban areas has recently increased (10), which could increase the risk for human exposure to CCHFV. Developing a robust surveillance system for detecting human cases and monitoring CCHFV infection in peri-urban dromedaries is essential for early detection of the risk and implementation of preventive measures.
Dr. Guidoum is a researcher at the Ibn Khaldoun University, Zaârour, Algeria. His current research focuses on emerging infectious diseases in the livestock/wildlife interface in desertic areas in North Africa.
Acknowledgments
We thank Boughalim Karim, Miloudi Abdellatif, Elbouti Khamra, Selami Zakaria, Madani Tallal, Youcef Maamra, Belgot Hamza, Abdeldjalil Ayadi, Ghedjmis Seifeddine, Sotri Hicham, Larbi Mohammed, the Golden Slaughterhouse owner in El Oued, the veterinary officer workers, and the participants for their contribution to the study.
L.C.-F. was funded through the 2022 FI Scholarship, Departament de Recerca i Universitats, Generalitat de Catalunya, Spain (FI_B 00723). The authors declare no competing interests.
References
- Estrada-Peña A, de la Fuente J. The ecology of ticks and epidemiology of tick-borne viral diseases. Antiviral Res. 2014;108:104–28. DOIPubMedGoogle Scholar
- Temur AI, Kuhn JH, Pecor DB, Apanaskevich DA, Keshtkar-Jahromi M. Epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Africa—-underestimated for decades. Am J Trop Med Hyg. 2021;104:1978–90. DOIPubMedGoogle Scholar
- Kautman M, Tiar G, Papa A, Široký P. AP92-like Crimean-Congo hemorrhagic fever virus in Hyalomma aegyptium ticks, Algeria. Emerg Infect Dis. 2016;22:354–6. DOIPubMedGoogle Scholar
- Faye B. How many large camelids in the world? A synthetic analysis of the world camel demographic changes. Pastoralism. 2020;10:25. DOIGoogle Scholar
- Arino O, Gross D, Ranera F, Leroy M, Bicheron P, Brockman C, et al. GlobCover: ESA service for global land cover from MERIS. 2007 IEEE Int Geosci Remote Sens Symp. 2007;2412–5.
- Schulz A, Barry Y, Stoek F, Ba A, Schulz J, Haki ML, et al. Crimean-Congo hemorrhagic fever virus antibody prevalence in Mauritanian livestock (cattle, goats, sheep and camels) is stratified by the animal’s age. PLoS Negl Trop Dis. 2021;15:
e0009228 . DOIPubMedGoogle Scholar - Bouaicha F, Eisenbarth A, Elati K, Schulz A, Ben Smida B, Bouajila M, et al. Epidemiological investigation of Crimean-Congo haemorrhagic fever virus infection among the one-humped camels (Camelus dromedarius) in southern Tunisia. Ticks Tick Borne Dis. 2021;12:
101601 . DOIPubMedGoogle Scholar - Bedda H, Adamou A, Bouammar B, Babelhadj B. The decline of camel production systems in Algerian Northern Sahara—case of the basin of Ouargla, the M’zab and the Ziban [in French]. Livest Res Rural Dev. 2019;31:44.
- Messina JP, Pigott DM, Golding N, Duda KA, Brownstein JS, Weiss DJ, et al. The global distribution of Crimean-Congo hemorrhagic fever. Trans R Soc Trop Med Hyg. 2015;109:503–13. DOIPubMedGoogle Scholar
- Mammeri A, Kayoueche FZ, Benmakhlouf A. Peri-urban breeding practice of one-humped camel (Camelus Dromedarius) in the Governorate of Biskra (Algeria); a new option. Journal of Animal Production Advances. 2014;4:403–15.
Figure
Table
Cite This ArticleOriginal Publication Date: October 31, 2023
Table of Contents – Volume 29, Number 12—December 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Oscar Cabezón, Departament de Medicina i Cirurgia Animals, Universitat Autònoma de Barcelona, Edifici V, 08193 Bellaterra, Spain
Top