Volume 29, Number 12—December 2023
Dispatch
Surveillance for Soil-Transmitted Helminths in High-Risk County, Mississippi, USA
Abstract
Recent reports of hookworm infection in Alabama, USA, has prompted surveillance in Mississippi, given the states’ similar environmental conditions. We collected stool specimens from 277 children in Rankin County, Mississippi. Kato–Katz microscopic smear, agar plate culture, and quantitative PCR indicated no soil-transmitted helminths. Nevertheless, further surveillance in other high-risk Mississippi counties is warranted.
Soil-transmitted helminths (STHs) are endemic in resource-limited settings worldwide. Because of its subtropical climate and socioeconomic factors, the southeastern United States historically has been at elevated risk for STH diseases. Because of improved sanitation and economic development, hookworm and other STHs were assumed to be eliminated from the US South (1). Mississippi has many areas with poor sanitation, but STHs have not been reportable since 1984 (2). Locally, expertise in clinical microscopic methods for STH diagnosis is lacking. At the University of Mississippi Medical Center (Jackson, MS, USA), stool samples are sent to the Mayo Clinic (Rochester, MN, USA), for routine ova and parasite examinations.
A recent report of suspected locally acquired cases of strongyloidiasis and hookworm in Alabama (3) initiated a parallel STH surveillance program in historically high-prevalence counties of Mississippi. After review of regions with high-risk criteria, including those with the soil type most conducive to human hookworm transmission (i.e., loamy soil) (4), review of Rockefeller Sanitary Commission data of areas with historically high prevalence, and review of sanitation data with the Mississippi Department of Health, we identified Rankin County as high risk. During 1910–1914, the Rockefeller Sanitary Commission found a very high prevalence of hookworm infection (42.1%) in Rankin County (5). The last formal surveillance study in Mississippi (1932–1933) found the prevalence in this county had decreased to 3.8% (5).
We conducted a cross-sectional study in which we recruited parents and guardians of children in Rankin County at health fair and community events (February 2020–September 2021) and asked participants to submit 3 stool specimens per participating child (Appendix). Participants submitted specimens directly to designated clinics or drop sites, where study personnel collected the specimens. Because of the connection between STH and poverty (3), we targeted areas within the county that had higher deprivation indices (https://www.neighborhoodatlas.medicine.wisc.edu).
We preserved ≈250-mg aliquots of fresh stool specimens in 70% ethanol at room temperature. For DNA extraction, we washed the specimens once in phosphate-buffered saline, followed by freezing at –80°C for 30 min, snap-thawing at 100°C for 10 min, then bead beating for 1 min with zirconium beads (Benchmark, https://www.benchmarkscientific.com) instead of the kit-supplied beads. Otherwise, we performed DNA extraction according to the manufacturer’s instructions. We processed the first 128 specimens by using the SurePrep Soil DNA Isolation Kit (Fisher Scientific, https://www.fishersci.com). This kit was discontinued by the manufacturer during the study, so we validated and processed the remaining 660 specimens by using the PowerFecal ProDNA kit (QIAGEN, https://www.qiagen.com) (6). We immediately stored DNA extracts at –80°C and sent them to the Division of Parasitic Diseases and Malaria, Center for Global Health, at the Centers for Disease Control and Prevention for quantitative PCR (qPCR) analysis.
Where specimen volume allowed, we performed microscopic analysis by using saturated sodium nitrate (NaNO3) centrifugal flotation as previously described but with a 500 × g (instead of 3,000 × g) centrifugation step (7). We performed Kato–Katz microscopy as previously described (8). We prepared Koga agar plate cultures (APCs) and inoculated as previously described (9). We sealed the plates with parafilm, incubated them at 28°C, and checked for larval tracks on days 3 and 5.
We quality-control tested DNA extracts for PCR inhibitors by using a human cytochrome B qPCR (10). We tested DNA samples without inhibition by using multiparallel qPCR specific for Necator americanus, Ancylostoma duodenale, Trichuris trichiura, Strongyloides stercoralis (11), and Ascaris lumbricoides (12). We considered a cycle threshold value <40 to be positive. We incorporated positive (genomic DNA from worms) and negative (water and DNA extracted from STH-free stool specimens) controls into each qPCR run.
We collected data regarding risk factors for STH by using case report forms from parents or guardians representing 354 children 2–18 years of age at the time of enrollment. The median age of children enrolled was 8 years (interquartile range 5.0–11.5 years); 55.4% were boys and 44.6% girls; 78.2% were White and 16.9% Black (US Census data for Rankin County [https://www.census.gov/quickfacts/rankincountymississippi] indicate the population is 74.5% White and 22.5% Black) (Table 1). Most (94.6%) reported non-Hispanic ethnicity, although US Census data indicate 72.2% are non-Hispanic in this county (Table 1). According to survey responses, 12.5% had traveled outside the United States in the previous 5 years, 7% had prior treatment for an intestinal parasite (80% had treatment for Enterobius vermicularis pinworm), most (89.7%) children had some contact with soil, and all had a flushable toilet (Table 2; Appendix Table [data for children for whom stool specimens were received]).
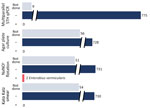
We received 784 stool specimens from 277 of the 354 survey respondents, representing 129 households (Figure). Three specimens taken over 3 days were submitted by 245 participants, 15 submitted only 2 specimens, and 17 submitted a single specimen. Laboratory processing was performed within 72 hours of specimen collection for 98% of specimens received. Sufficient specimen was present to perform NaNO3 flotation microscopy on 731 specimens. We performed Kato–Katz microscopic analysis on 730 specimens and conducted APC on 728 specimens. All NaNO3 flotations, Kato–Katz microscopy, and APC tests showed no STH. Two participants (0.6%) were positive in 1 of 3 specimens each for Enterobius vermicularis eggs by NaNO3 flotation only.
We extracted DNA from all specimens and subjected them to qPCR analysis. Negative results in a DNA quality-control assay excluded 9 specimens (1.15%) from further qPCR analysis. We subjected the remaining 775 DNA extracts to multiparallel STH qPCR, all of which were negative. Of note, the 9 excluded specimens were all negative by NaNO3, Kato–Katz microscopic analyses, and APC.
Our findings suggest the absence of STH infections among the children surveyed in Rankin County, Mississippi. We suspect that our survey results may not have captured sanitation and hygiene data: a limitation of our survey design was that it did not enquire about the endpoint of flush toilets, so any household effluent released nearby through straight pipes was possibly not detected by our survey tool. Furthermore, a stigma associated with having substandard sanitation or fear of ramifications for the need to install appropriate sanitation may have limited the veracity of some responses. However, our laboratory data are consistent with our prior surveillance in the Mississippi Delta (10) and work with postdiagnostic specimens in Mississippi (2), which demonstrated no human hookworm, Ascaris, or Trichuris spp. infections by microscopic analysis or qPCR. Rare S. stercoralis infections were detected previously in Mississippi residents by serologic analysis, but whether those infections were autochthonously acquired, travel-acquired, or chronic or persistent infections many decades after exposure is unclear (2). We have observed sporadic cases of Ascaris hookworm infections in Mississippi (C. Hobbs, unpub. data), usually in association with pig farming, and those cases may therefore represent zoonotic acquisition of A. lumbricoides pig genotype. Both hookworm and S. stercoralis worms are common in children in STH-endemic areas globally but increase in prevalence with age (13,14). Further surveillance of adults might identify rare STH infections, particularly persistent cases of strongyloidiasis. Even though 12.5% of respondents in our investigation had traveled outside of the United States, none harbored travel-acquired helminthic infections.
The prevalence of enterobiasis was much lower in our study compared with the most recent surveillance from the United States, which reported prevalence of 11.6%–38.9% in southern California elementary schools in the early 1980s (15). Our results may represent actual lowered prevalence or may be attributable to the poor sensitivity of NaNO3 flotation for E. vermicularis detection.
Further study is needed in several other counties that have had historically high levels of hookworm infection. However, considered in the context of other recent surveillance studies (2,10), it is becoming increasingly likely that continued transmission of STH is not occurring in this high-risk county in Mississippi.
Dr. Bradbury is a parasitologist and microbiologist with expertise in laboratory diagnostics and epidemiology. He maintains a research program in parasitic diseases, zoonoses, and parasite diagnostics. Dr. Hobbs is a professor of pediatric infectious disease and attending physician at Children’s of Mississippi and professor of cell and molecular biology at University of Mississippi Medical Center, Jackson, Mississippi, USA. Her research interests include parasitic diseases in children in resource-limited settings.
Acknowledgments
We thank the patients, their parents, and guardians for their participation in this study. We also thank Susan P. Montgomery, Emily Dodd, and Anne Straily.
This work was funded by the University of Mississippi Medical Center and the Centers for Disease Control and Prevention.
Author contributions: R.S.B. cowrote the draft paper, assisted in study design, and provided technical support to University of Mississippi Medical Center. C.V.H. and J.M.W. performed data analysis. G.S., J.M.W., K.P., and I.A. performed microscopic analysis, agar plate culture, and all DNA extractions. L.M., L.M., M.M., C.S., and L.H. conducted specimen collection. P.B. provided input into manuscript writing. Y.Q. analyzed PCR data and reviewed the manuscript. E.R. performed PCR. C.V.H. obtained funding and specimens, designed the study, and supervised and managed ethical approvals at University of Mississippi Medical Center, supervised and aided in specimen collection, salt flotation microscopic analyses, and DNA extraction and cowrote the paper. All authors reviewed and approved the final draft of the paper.
References
- Bleakley H. Disease and development: evidence from hookworm eradication in the American South. Q J Econ. 2007;122:73–117. DOIGoogle Scholar
- Bradbury RS, Lane M, Arguello I, Handali S, Cooley G, Pilotte N, et al. Parasitic disease surveillance, Mississippi, USA. Emerg Infect Dis. 2021;27:2201–4. DOIPubMedGoogle Scholar
- McKenna ML, McAtee S, Bryan PE, Jeun R, Ward T, Kraus J, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623–8. DOIPubMedGoogle Scholar
- Mississippi Agricultural and Forestry Experiment Station. Information sheet 1278: soil resource areas of Mississippi. 1977 [cited 2023 Mar 23]. https://www.mafes.msstate.edu/publications/information-sheets/i1278.pdf
- Keller A, Leathers W, Ricks H. An investigation of the incidence and intensity of infestation of hookworm in Mississippi. Am J Epidemiol. 1934;19:629–56. DOIGoogle Scholar
- Bradbury R, Inagaki K, Singh G, Agana U, Patterson K, Malloch L, et al. A pilot comparison of fixatives for hookworm real-time polymerase chain reaction. Am J Trop Med Hyg. 2022;108:335–9. DOIPubMedGoogle Scholar
- Inpankaew T, Schär F, Khieu V, Muth S, Dalsgaard A, Marti H, et al. Simple fecal flotation is a superior alternative to guadruple Kato Katz smear examination for the detection of hookworm eggs in human stool. PLoS Negl Trop Dis. 2014;8:
e3313 . DOIPubMedGoogle Scholar - Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400.PubMedGoogle Scholar
- Koga K, Kasuya S, Khamboonruang C, Sukavat K, Nakamura Y, Tani S, et al. An evaluation of the agar plate method for the detection of Strongyloides stercoralis in northern Thailand. J Trop Med Hyg. 1990;93:183–8.PubMedGoogle Scholar
- Bradbury RS, Arguello I, Lane M, Cooley G, Handali S, Dimitrova SD, et al. Parasitic infection surveillance in Mississippi Delta children. Am J Trop Med Hyg. 2020;103:1150–3. DOIPubMedGoogle Scholar
- Pilotte N, Papaiakovou M, Grant JR, Bierwert LA, Llewellyn S, McCarthy JS, et al. Improved PCR-based detection of soil transmitted helminth infections using a next-generation sequencing approach to assay design. PLoS Negl Trop Dis. 2016;10:
e0004578 . DOIPubMedGoogle Scholar - Pilotte N, Maasch JRMA, Easton AV, Dahlstrom E, Nutman TB, Williams SA. Targeting a highly repeated germline DNA sequence for improved real-time PCR-based detection of Ascaris infection in human stool. PLoS Negl Trop Dis. 2019;13:
e0007593 . DOIPubMedGoogle Scholar - Loukas A, Hotez PJ, Diemert D, Yazdanbakhsh M, McCarthy JS, Correa-Oliveira R, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:16088. DOIPubMedGoogle Scholar
- Khieu V, Schär F, Forrer A, Hattendorf J, Marti H, Duong S, et al. High prevalence and spatial distribution of Strongyloides stercoralis in rural Cambodia. PLoS Negl Trop Dis. 2014;8:
e2854 . DOIPubMedGoogle Scholar - Wagner ED, Eby WC. Pinworm prevalence in California elementary school children, and diagnostic methods. Am J Trop Med Hyg. 1983;32:998–1001. DOIPubMedGoogle Scholar
Figure
Tables
Cite This ArticleOriginal Publication Date: November 14, 2023
Table of Contents – Volume 29, Number 12—December 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Charlotte V. Hobbs, Department of Pediatrics, Division of Infectious Disease, Department of Cell and Molecular Microbiology, University of Mississippi Medical Center, Batson Children's Hospital, 2500 N State St, Jackson, MS 39202, USA
Top